No Of Moles Formula
Finally multiply all the moles by the same number to get whole numbers rather than fractions. This calculation is shown in step 2 below.


4 Ways To Calculate Molarity Wikihow

Mole Conversion Worksheet And Activity Iteachly Com
Cu 259 S 127 O 574 H 4.
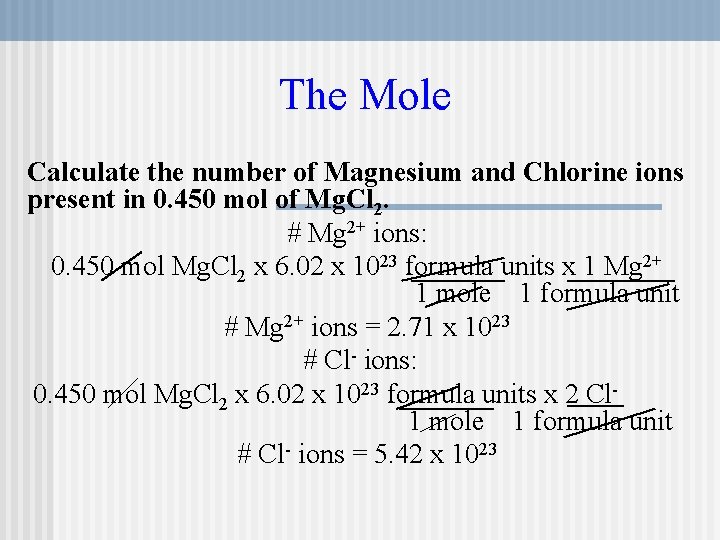
No of moles formula. 1 A compound is found to contain 8563 C and 1437 H by mass. What is its molecular formula. A chemical formula can be determined from the mass of each element present in a.
In another experiment its molar mass is found to be 561 gmol. A small mammal that is almost blind has dark fur and lives in passages that it digs. A chemical formula is a usually a whole number ratio showing the relative numbers of moles of each element present.
Empirical Formula Example Calculation A compound is analyzed and calculated to consist of 135 g Ca 108 g O and 0675 g H. Likewise 10 mole of H 2 O is composed of 20 moles of hydrogen and 10 mole of oxygenWe can also work backwards from molar ratios because if we know. What is the molecular formula of the compound.
Using a calculator divide the number of grams by the molar mass. The molar mass of the compound is known to us M 168096 g mol 1. The number of moles of a substance in a sample is obtained by dividing the mass of the sample by the molar mass of the compound.
For example imagine you have 2 g of NH 4 2 S and you want to convert it to moles. The molar mass of a substance depends not only on its molecular formula but also on the distribution of isotopes of each. What is the empirical formula of this compound of copper.
Find the empirical formula of the compound. Divide 2 by 6817 and you have 00293 moles of NH 4 2 S. Then divide each elements moles by the smallest number of moles in the formula to find their relative weights.
UA008883 Workbook for GCE students Moles Formulae and Equations 3. To convert between mass and number of moles you can use the molar mass of the substance. The molecular mass of NH 4 2 S is 6817gmol.
This equation formula or expression can be re-arranged to find. Therefore the empirical formula is C 3 H 2 NO 2. Moles of solute given molarity and volume of solution.
The entire group 1 metal can react with oxygen to form metal oxide. No ads no money for us no free stuff for you. Determining Empirical Formulas.
The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. 3 The Empirical Formula of a Compound. Grams to Moles Formula.
To calculate or find the grams to moles or moles to grams the molar mass of each element will be used to calculate. N c V volume of solution given moles of solute and molarity. Although there is no physical way of measuring the number of moles of a compound we can relate its mass to the number of moles by using the compounds molar mass as a direct conversion factor.
Relative atomic mass molecular mass and formula mass have no units. Multiply all of the subscripts in the empirical formula by this ratio to get the subscripts for the molecular formula. Grams and moles are two units to express the amount of matter in a sample.
In this topic we will discuss the formula for the Molar Concentration formula with examples. It has the following percentages by mass. Etc or of Items atomsmolec of Given reactant or product Moles of Given Mole Ratio from the.
We can write the empirical formula by placing the numbers as the subscript to the elements symbols. For example 100 g of water is about 5551 mol of water. Moles mass molar mass or n m M Substitute the values into the equation and solve to find moles of oxygen gas.
An empirical formula tells us the relative ratios of different atoms in a compound. Reaction of Group 1 Elements. Calculating the molar concentration of a solution is a comparatively straightforward process.
The ratios hold true on the molar level as well. Thus H 2 O is composed of two atoms of hydrogen and 1 atom of oxygen. We find that there are 0138 moles of pentane 0116 moles of hexane and 0128 moles of benzene.
16 grams of Oxygen has 05 moles. Figuring out the empirical formula from a molecules. We will determine how many moles of a substance we have and then divide it by the volume of the solution.
2 moles 3 moles X 90 g O2 1 mole O2 2 mole KClO3 1225 g KClO3 2297 g KClO3 32 g O2 3 moles O2 1 mole KClO3 I II III IV CHEMISTRY STOICHIOMETRY Mass gkg etc Volume L mL. The empirical mass of the compound is obtained by adding the molar mass of individual elements. Therefore to convert moles of H 2 O to moles of H we must multiply moles of H 2 O by the ratio 2 mol H1 mol H 2 O.
120 g carbon is about 1 mole of carbon. To find the molar mass of gaseous compounds use our molar mass of gas calculator. Moles n 1245 g 3200 g mol-1 3891 mol 4 significant figures are justified Worked Example.
No calculator allowed Solution. We can find the total number of moles by taking the sum of all the moles. Their dental formula varies but is usually somewhere near 43124 3134 2.
As a result we can say that one mole of H 2 O will always contain 2 moles of H and 1 mole of O. P 2 O 5. The mole calculator uses grams to moles formula to get actual results.
The number of moles for each is found by dividing its mass by its respective molecular weight. An oxide of nitrogen has 696 by mass of oxygen. 013801160128 0382 total moles.
Apply formula as shown below or place the values in moles to atoms calculator. The teeth of the marsupial moles are degenerate and bear no resemblance to polyprotodont or diprotodont teeth. Mole 1632 05.
On analysis a compound with molar mass 60 gmol was found to contain 120 g of carbon 20 g of hydrogen and 160 g of oxygen. Please do not block ads on this website. Reaction with Oxygen.
The result is the number of moles in your element or compound. Next convert the grams to moles by dividing 293 grams by the atomic weight of sodium which is 2299 grams to get 1274. Instead you must use atomic mass values and the chemical formula to do the conversion.
Formula from Mass Composition. Use moles to grams converter to verify the number of moles in the above example. ONE-SCHOOLNET Periodic Table.
What is the empirical formula. Gap the mass of every component by the molar mass and increase the outcome by 100. CuSO 9 H 10.
Moles are units used to measure substance amount. But since we are interested in hydrogen we ignore the oxygen. There is no conversion formula between the two units.
Write the mathematical equation mathematical formula. V n c.

Mole Calculations Explained Formula Mass And Mole Calculations
Analogies

Chemistry Relation Between Mole Avogadro Number And Mass Atoms And Molecules Part 8 Youtube
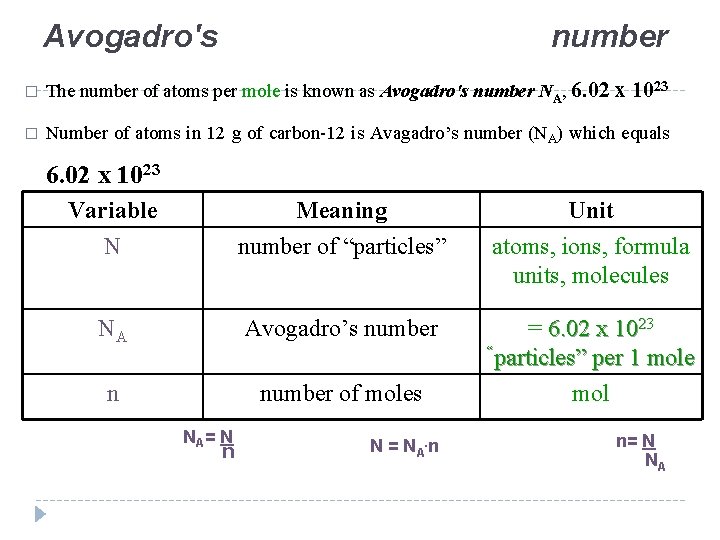
Lecture 5 The Mole Avogadros Number The Mole

Prelim Chemistry Introduction To The Mole Concept Art Of Smart

How You Measure How Much You Can Measure
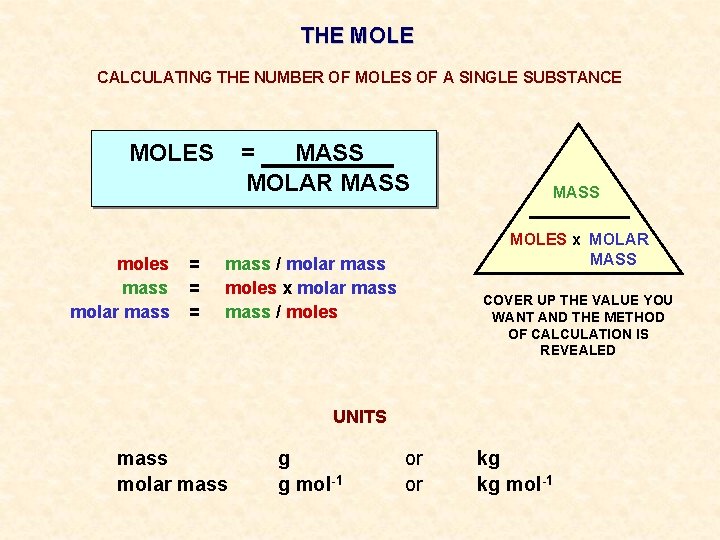
The Mole Contents What Is A Mole And
The Mole And Avogadro S Number Video Khan Academy



0 Response to "No Of Moles Formula"
Post a Comment