Ethyl Ethanoate Formula
Is och2ch3 a strong base. Add 15 mL of alcohol eg ethanol and 10 mL carboxylic acid eg glacial acetic acid to a 50 mL round bottom flask.
Igcse Chemistry 2017 4 39c Know That Ethyl Ethanoate Is The Ester Produced When Ethanol And Ethanoic Acid React In The Presence Of An Acid Catalyst

Methyl Acetate Wikipedia
.jpg)
Distinguishing Between The Isomers Of Ethyl Acetate Butyric Acid And Isobutyric Acid Using Proton Nmr Spectroscopy
It is one of the ingredients responsible for the characteristic smell of a banana flower.

Ethyl ethanoate formula. Ethanoate comes from ethanoic acid. SOLUTION 10 IN CCl4 FOR 3800-1300 10 IN CS2 FOR 1300-650 10 IN CCl4 FOR 650-250 CM-1 VERSUS SOLVENT. It is also called as ethyl ethanoate commonly abbreviated EtOAc or EA.
The formula for ethyl ethanoate is. In this case the hydrogen in the -COOH group has been replaced by an ethyl group. A common ester - ethyl ethanoate.
5OH Ethanoate ion Acetate ion CH 3CO 2-Propanol C 3H 7OH Propanoate ion Propionate ion C 2H 5CO 2-Special Names Formula Butanol C 4H 9OH Butanoate ion Butyrate ion C 3H 7CO 2-Benzene C 6H 6 Pentanol C 5H 11OH Pentanoate ion-Valerate ion C 4H 9CO 2 Toluene C 6H 5CH 3 Most Common Formula Representations All represent ethanol Example C 2H 6O CH. If it were hydrogen atom the compound would be a carboxylic acid Figure. As the esters get bigger the smells tend towards artificial fruit flavouring - pear drops for example.
The ethanoate bit comes from ethanoic acid. Here the hydrogen in the -COOH group is replaced by an ethyl group. We provide custom synthesis and contract manufacturing from milligrams to metric tonnes.
A mixture of ethanoic acid ethanol and concentrated sulfuric acid is gently heated by either a water bath or an electric. Esters have the general formula RCOOR where R may be a hydrogen atom an alkyl group or an aryl group and R may be an alkyl group or an aryl group but not a hydrogen atom. There is a negative charge on oxygen atom in the CH 3 CH 2 O-.
Chat now for more business. Alkoxide ion is a strong alkali. A 4-ethyl-cis-3-octene b 4-ethyl-trans-3-octene.
Notice that the ester is named the opposite way around from the way the formula is written. In this case the hydrogen in the -COOH group has been replaced by an ethyl group. Try this class practical to prepare the ester ethyl benzoate on a microscale by warming ethanol and benzoic acid.
Jiangyin Healthway International Trade Co Ltd is a professional company main engaged in manufacturing and exporting aroma chemicals food additives cosmetic ingredient pharmaceutical intermediates. Propyl acetate also known as propyl ethanoate is a chemical compound used as a solvent and an example of an ester. Slowly add about 1 mL of concentrated sulfuric acid and a few boiling chips which will prevent bumping 4.
The formula for ethyl ethanoate is. So this can take H ion very easily to show strong basic characteristics. This clear colorless liquid is known by its characteristic odor of pears.
Ethyl Acetate Formula - Ethyl Acetate is commonly known as ethyl ethanoate is an important chemical compound. The correct name for the compound given below is. The most commonly discussed ester is ethyl ethanoate.
Is och2ch3 a strong base. Small esters like ethyl ethanoate smell like typical organic solvents ethyl ethanoate is a common solvent in for example glues. In the early stages of the development of organic chemistry there was no systematic way of assigning names to organic compoundsCompounds were usually named after the source from which they are obtained.
Contact China Manufactory Wuhan Boyuan Import Export Co LTD for the product Ethyl acetate CAS. The preparation of ethyl ethanoate Method. Ethenyl ethanoate and an acrylic ester for example methyl 2-methylpropenoate are then co-polymerized to form a random array in which these groups link into a linear chain.
The steps in the procedure for the synthesis of the ester ethyl acetate ethyl ethanoate are given below. Ethyl Acetate is a most familiar ester of ethanol which you can easily remember by its regular use in our daily life. The naming of esters can be confusing to students who are new to organic chemistry because the name is the opposite of the order in which the formula is written.
A 2-methyl-1-butene b 2-ethyl-1-propene c 2-ethyl-1-pentane d 3-methyl-2-butene e pentene 9. Other acrylic esters used as co-monomers with ethenyl ethanoate are ethyl propenoate butyl propenoates or a co-polymer of butyl propenoate and methyl 2-methylpropenoate. What is Common Name of Chemical Compounds.
In this case the ester is usually named in the opposite way around from the way the formula is written. Diltiazem is a 5-2-dimethylaminoethyl-2-4-methoxyphenyl-4-oxo-2345-tetrahydro-15-benzothiazepin-3-yl acetate in which both stereocentres have S configuration. The Ethyl Acetate chemical formula is CH3COOCH2CH3 and its condensed formula is C4H8O2 and its molar mass is 8811gmol.
Notice that the ester is named the opposite way around from the way the formula is written. Try this demonstration to determine the formula of water through the reaction of copperII oxide with hydrogen. For example the name formic acid was derived from formicus meaning red ants because the compound was obtained from red ants.
The common names of chemical compounds do not follow special types of rules as in IUPAC names. Especially on aroma chemicals as the major. R is an alkyl group such as methyl ethyl propyl and more.
The ethanoate bit comes from ethanoic acid. Ethyl acetate systematically ethyl ethanoate commonly abbreviated EtOAc ETAC or EA is the organic compound with the formula CH 3 COOCH 2 CH 3 simplified to C 4 H 8 O 2This colorless liquid has a characteristic sweet smell similar to pear drops and is used in glues nail polish removers and in the decaffeination process of tea and coffee. Select the best name for.
GRATING CHANGES AT 2000 630 CM-1. PERKIN-ELMER 521 GRATING Instrument parameters. 141-78-6 CAS NO141-78-6 99 Colorless liquid BY BY.
On a larger scale. In general common names are easy to remember and convenient to use since the naming method does not consider the magnitude of the molecule functional groups or the molecular compositionIn some occasions some chemicals have a. A C n H 2n2 b C n H 2n c C n H 2n-2 d C n H n2 e C n H n.
The most commonly discussed ester is ethyl ethanoate. In this case ethanoate part comes from ethanoic acid and the ethyl part comes from the ethyl group on the end. A calcium-channel blocker and vasodilator it is used as the hydrochloride in the management of angina pectoris and hypertensionIt has a role as a calcium channel blocker a vasodilator agent and an antihypertensive agent.
Also akoxide ion are good nucleophiles and ligands. The general formula for noncyclic alkenes is. In the case of ethyl ethanoate for example the ethyl group is listed before the name.
This reaction is a. 0012 0012 AND 0010 CM CsBr CELL Resolution. Due to this fact it is commonly used in fragrances and as a flavor additive.
You can find the ethyl ethanoate formula below. Methyl acetate is an acetate ester resulting from the formal condensation of acetic acid with methanolA low-boiling 57 colourless flammable liquid it is used as a solvent for many resins and oils. Nomenclature of Organic Compounds.
Sodium ethanoate stalagmite.

Y12 Organic 3 At Gcse Ethanol Ethanoic Acid And Ethyl Ethanoate Youtube
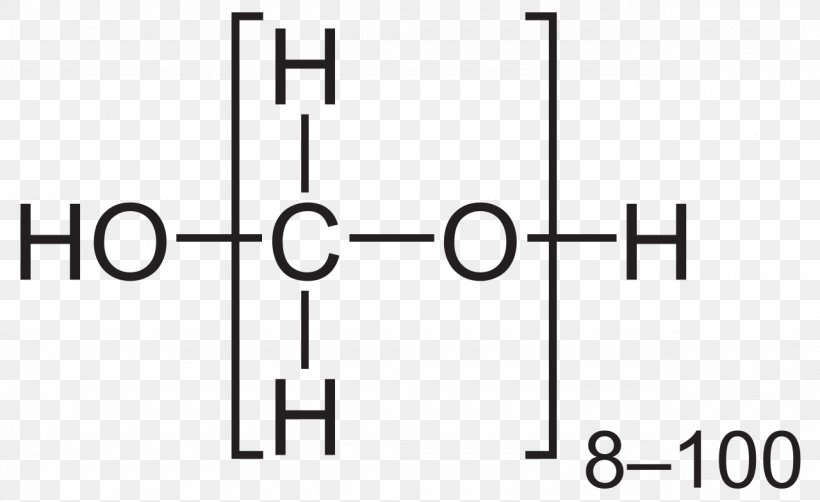
Ethyl Acetate Ethyl Group Structural Formula Chemistry Png 1280x785px Ethyl Acetate Acetate Acetic Acid Area Black

Ethylene Vinyl Acetate Wikipedia
Ethyl Acetate Molecule Of The Month March 2003 Html Version

4 40 Triple Only Understand How To Write The Structural And Displayed Formulae Of Ethyl Ethanoate Tutormyself Chemistry

Esters Edexcel Igcse Chemistry Revision Notes

Ethyl Acetate American Chemical Society
Igcse Chemistry 2017 4 40c Understand How To Write The Structural And Displayed Formulae Of Ethyl Ethanoate
0 Response to "Ethyl Ethanoate Formula"
Post a Comment