Charge Of Proton
There are 2 types of electric charge. It has a neutral charge also known as a charge of zero.

Protons Neutrons And Electrons Chapter 4 The Periodic Table Bonding Middle School Chemistry
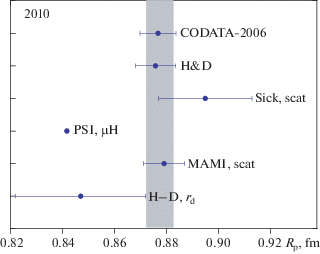
Determination Of The Proton Charge Radius By Different Methods Springerlink

Calculated And Experimental Proton Charge Neutron And Matter Radii Download Table
The proton always has a or positive charge.

Charge of proton. If the charge is negative add the amount of charge to the atomic number to get the number of electrons. Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core. One or more protons are present in the nucleus of every atom.
It has long been considered to be a stable particle but recent developments of grand unification models have suggested that it might decay with a half-life of about 10 32 years. The force on a negative charge is in exactly the opposite direction to that on a positive charge. Then setting the neutron speed equal to those proton speeds he used the above energy expression to get a.
Charge Of Proton Proton a stable subatomic particle that has a positive charge equal in magnitude to a unit of electron charge and a rest mass of 167262 10 27 kg which is 1836 times the mass of an electron. Neutrons do not have a net electric charge so the number of neutrons does not matter in the calculation. The tiny proton will be buried within the lone pair and will form a shared-electron coordinate bond with it creating a hydronium.
The protons atomic mass unit is 1. The charge on the ion tells you the number of electrons. If the charge of an entire atom is 0 or neutral there are equal numbers of positive and negative charges.
Mass of proton can be measured using the units kg MeVc and u AMU. Very light hard-to-detect particle. A proton has a mass of 16721027 kg.
In physics charge also known as electric charge electrical charge or electrostatic charge and symbolized q is a characteristic of a unit of matter that expresses the extent to which it has more or fewer electrons than protonsIn atoms the electron carries a negative elementary or unit charge. Neutral atoms have equal numbers of electrons and protons. A hydrogen ion that has lost its electron written as H.
Atoms consist of smaller particles called protons neutrons and electronsHere is the definition of a proton its electric charge where its found in the atom and a collection of proton facts. The electric charge influence other electric charges with electric force and influenced by the other charges with the same force in the opposite direction. Charge thus exists in natural units equal to the charge of an electron or a proton a fundamental physical constant.
Atoms contain three types of subatomic particles called protons neutrons and electrons. The center of an atom that contains the protons and neutrons. Charge comes in multiples of an indivisible unit of charge represented by the letter e.
If an ion has a 2 charge like Zn 2 this means there are two more protons than electrons. What is electric charge. Positive charge Positive charge has more protons than electrons NpNe.
You have more protons. Electric charge generates electric field. The mass of a.
A proton has a positive charge. It is the lightest and most stable baryon having a charge equal in magnitude to that of the electron a spin of ½ and a mass of 1673 10-27 kg. Protons carry a positive charge and are found in the nucleus of an atom.
They are a necessary. How did Rutherford figure out the structure of the atom without being able to see it. In physics the proton-to-electron mass ratio μ or β is simply the rest mass of the proton a baryon found in atoms divided by that of the electron a lepton found in atoms.
Subatomic particles that have a neutral charge and are located. The two types of charge are equal and opposite. Protons are only engaged in processes that take place inside the nucleus.
If the charge is positive subtract that number from the atomic number to get the number of electrons. The number of protons of an atom cannot change via any chemical reaction so you add or subtract electrons to get the correct charge. Proton is a new tool released by Valve Software that has been integrated with Steam Play to make playing Windows games on Linux as simple as hitting the Play button within Steam.
Because the force is always perpendicular to the velocity vector a pure magnetic field will not accelerate a charged particle in a single direction however will produce circular or helical motion a concept explored in more detail in future sections. Underneath the hood Proton comprises other popular tools like Wine and DXVK among others that a gamer would otherwise have to install and maintain themselves. The charge of the proton is positive that is 1e elementary charge.
Smash two protons together to make deuterium. The part of a molecule that has a positive electric charge. These things have the same size charge but the sign is different.
Like an electron but with positive charge neutrino. The third particle is the neutron. Neutrally charged particle in an atom.
Negatively charged particle in an atom. It resides in the atomic nucleus. Proton location is inside the nucleus.
In other words charge comes in multiples of the charge on the electron or the proton. P is the symbol for protons. A neutron is either neutrally charged or uncharged.
Protons are found within the nucleus of atoms. A proton has a charge of e while an electron has a charge of -e. Log in or create an account.
Protons together with electrically neutral particles called neutrons make up all atomic nuclei except for the hydrogen nucleus which consists of a single proton. A proton is a subatomic particle symbol p or p with a positive electric charge of 1e elementary charge and a mass slightly less than that of a neutronProtons and neutrons each with masses of approximately one atomic mass unit are jointly referred to as nucleons particles present in atomic nuclei. The resulting extraordinarily high charge density of the proton strongly attracts it to any part of a nearby atom or molecule in which there is an exess of negative charge.
Proton definition a positively charged elementary particle that is a fundamental constituent of all atomic nuclei. The proton carries a positive charge. The proton mass is slightly less than the neutron mass.
Mass of the proton is the sum of the mass of current quarks and the binding gluons. A protein found in the cell membrane that helps control what can enter or exit a cell. Along with neutrons protons make up the nucleus held together by the strong forceThe proton is a baryon and is considered to be composed of two up quarks and one down quark.
The process is called the Proton-Proton PP Chain and it operates inside the Sun and stars of similar mass. In the case of water this will be the lone pair unshared electrons of the oxygen atom. Positively charged particle in an atom.
A direct and convincing measurement of an electrons charge as a natural unit of electric charge was first made 1909 in the Millikan oil-drop experiment. Because this is a ratio of like-dimensioned physical quantities it is a dimensionless quantity a function of the dimensionless physical constants and has numerical value independent of the system of units namely. The symbol for neutrons is.
A proton is a subatomic particle with a positive charge. Assuming that the neutron mass was close to that of the proton Chadwick bombarded hydrogen atoms with his produced neutrons to learn the speed of the protons after the collisions.

Mass Of Neutron Definition And The Examples Of Neutron

Atomic Structure Vocabulary And Study Guide
Electric Charge Review Article Khan Academy

The Value Of Charge On A Proton Is Coloumbs

The Structure Of The Atom Boundless Chemistry
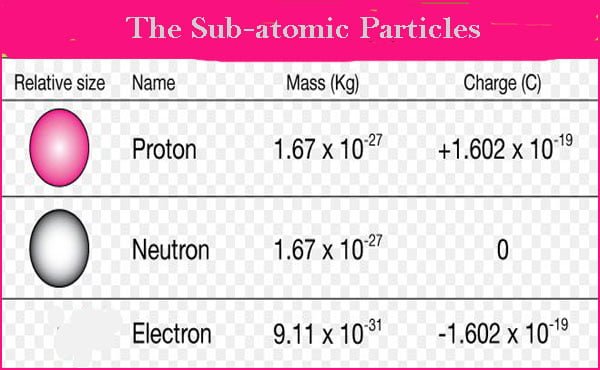
Charge Of Proton Along With Mass Charge And History Of It

Protons Structure Properties Expii

Proton Radial Charge Distribution Download Scientific Diagram

0 Response to "Charge Of Proton"
Post a Comment