Sodium Carbonate And Hydrochloric Acid
Sodium hydroxide dilute hydrochloric acid and phenolphthalein Method. Like the similar-sounding compound sodium bicarbonatebaking sodaits basic and can react with acids.

Solved Identify The Correct Equation And Net Ionic Equation Chegg Com

Sodium Carbonate Hydrochloric Acid Balanced Molecular And Net Ionic Equation Na2co3 Hcl Quizalize

Solved E The Balanced Equation For The Reaction Between Chegg Com
Sodium carbonate is more commonly known as washing soda.
Sodium carbonate and hydrochloric acid. This chemistry video tutorial explains how to predict the products of the reaction between Sodium Carbonate and Hydrochloric Acid. Add a small amount of sodium carbonate to the acid and seal the test tube with the rubber stopper. Carefully pour a small amount of hydrochloric acid into the other test tube.
Adding hydrochloricmuriatic acid to reduce total alkalinity. To avoid titration errors we boil titrated solution to remove. Industrial Environmental Social Links A standard solution of sodium carbonate can be used in the standardisation of hydrochloric acid solutions for analytical work.
Sodium carbonate is a salt of a weak acid. Now pour hydrochloric acid to it dropwise usjng a dropper till. Standardization 01 N Hydrochloric Acid with Sodium Carbonate.
Historically it was extracted from the ashes of plants growing in sodium-rich soils. Answer 1 of 14. Calcium carbonate CaCO 3 is a metal carbonate compound and reacts with hydrochloric acid HCl to produce carbon dioxide CO 2 calcium chloride CaCl 2 and waterYou can see carbon dioxide gas is released through the solution.
Hydrochloric acid is an important laboratory reagent and industrial chemical. Acetic acid acetic aldehyde acetone. Weight accurately 022 g of the dried Na2CO3 and transfer to a.
To write the net ionic equation for Na2CO3 HCl we follow three steps. Na 2 CO 3 2HCl 2NaCl CO 2 H 2 O. Take 30 ml of dilute sodium hydroxide solution in a conical flask and add a drop of phenolphthalein to it.
Take a burette and wash it with water. Calcium Carbonate and Hydrochloric Acid Reaction CaCO 3 HCl. HCl or hydrochloric acid is a strong acid that reacts with sodium carbonate.
Always handle it with care to prevent harm or injury. This is the list of all compounds with density tables present in the full database - it was actual on May 4 th 2005 as the list is growing it may be already incomplete. Sodium carbonate Na 2 CO 3 10H 2 O also known as washing soda soda ash and soda crystals is the inorganic compound with the formula Na 2 CO 3 and its various hydrates.
HAWKINS manufactures Sodium Hypochlorite Bleach in St. Yellow to pink Acid in burette. Answer 1 of 5.
All forms are white odourless water-soluble salts that yield moderately alkaline solutions in water. Hydrochloric acid solution may be titrated against sodium carbonate solution using methyl orange indicator. Hydrochloric acid also known as muriatic acid is an aqueous solution of hydrogen chloride chemical formula.
If you were to mix a dilute aqueous solution of NaOH with a dilute aqueous solution of HCl you would get a di. Check our FAQ section for more details. Take 05 oto 1 g of primary standard anhydrouso sdium carbonate Na2CO3 to a suitable dish or crucible and dry at 250C for 4 h.
Adding stabiliser cyanuric acid. Paul MN Camanche IA Dupo IL and Memphis TN. When titrated with hydrochloric acid carbonate decomposes yielding carbon dioxide and water.
Second we break the soluble ionic compou. These lumps are quite difficult to dissolve. Since calcium carbonate is relatively insoluble it tends to come out of solution.
Evolving carbon dioxide acidifies the solution and the end point in its presence is detected too early. Adding soda ash sodium carbonate to increase pH. It is a by-product of chlorine manufacture along with sodium hypochlorite and sodium hydroxide.
When weak base is titrated with a strong acid solution is slightly acidic at end point. Hawkins carries Sodium Hypochlorite in bulk and in multiple packaging options. Calcium bicarbonate entering with the feed water is broken down at boiler temperatures or reacts with caustic soda to form calcium carbonate.
Sodium silicate is used to react selectively with magnesium hardness. Sodium carbonate solution into the flask and add 2-3 drops of methyl orange indicator. Cool in a desiccator.
This includes stomach acid. H ClIt is a colorless solution with a distinctive pungent smell. Concentrations from 3-20 by weight sodium hypochlorite are available with 125 being a preferred strength for long term storage and ease of transport.
First we balance the molecular equation. Hydrochloric acid If less than 68 M but 27 M or more IRRITANT. Swirl the conical flask vigorously and while continuing to swirl add the hydrochloric acid solution.
Dilute hydrochloric acid Dilute acid may still cause harm in the eyes or in a If less than 27 M LOW HAZARD. It is classified as a strong acidIt is a component of the gastric acid in the digestive systems of most animal species including humans. If a weak acid is titrated with a strong base the solution is slightly basic because the salt formed will be hydrolysed to a certain extent.
Observe what happens to. It may irritate the eyes and respiratory system. If you were foolish enough to put solid NaOH into commercial hydrochloric acid and stir the heat generated would very likely splash a lot of corrosive material around.
Typical control measures to reduce risk Use the lowest concentration. It explains how to write. Place the other end of the delivery tube into the test tube containing the lime water.
Repeat the titration for a more accurate reading by adding in hydrochloric acid solution from the burette rapidly to within 2 cm 3 of the end point. Rinse the pipette with the given sodium carbonate solution and pipette out 20 ml of this solution in a washed titration flask. Adding dry acid sodium bisulphate to reduce total alkalinity.
The reaction produces large quantities of carbon dioxide gas water and table salt. Adding calcium chloride to increase calcium hardness. Rinse the burette with the given solution of hydrochloric acid and fill it with it.
Different values of the pure water 0 concentration density reflect the fact that the measurements were done in different temperatures. When you add a hydrochloric acid HCl solution to a solution of sodium carbonate Na 2 CO 3 the hydrogen ion in HCl switches places with one of the sodium ions in Na 2 CO 3 to produce sodium hydrogencarbonate also known as sodium bicarbonate baking soda and sodium chloride salt. Hydrochloric acid HCL is a clear colourless and pungent solution created by dissolving hydrogen chloride gas in water.
A Two-Stage Reaction When you add a hydrochloric acid HCl solution to a solution of sodium carbonate Na2CO3 the hydrogen ion in HCl switches places with one of the sodium ions in Na2CO3 to produce sodium hydrogencarbonate also known as sodium bicarbonate baking soda and sodium chloride salt. You need to take specific safety precautions when handling transporting and storing HCl and get medical help immediately if accidental contact occurs. Adding sodium bicarbonate to increase total alkalinity.
To prevent the formation of hard lumps of sodium carbonate. If HCl is limiting then it forms a buffer solution. Hydrochloric acid -- or HCl -- is an acid that is highly corrosive when concentrated.
Which Of The Following Gases Is Formed When Solid Sodiumcarbonate Reacts With Dilute Hydrochloric Acid A Hydrogen B Oxygen C Carbon Dioxide D Carbon Monoxide Snapsolve
1
What Do We Observe When Diluted Hydrochloric Acid Is Added To Sodium Carbonate Quora
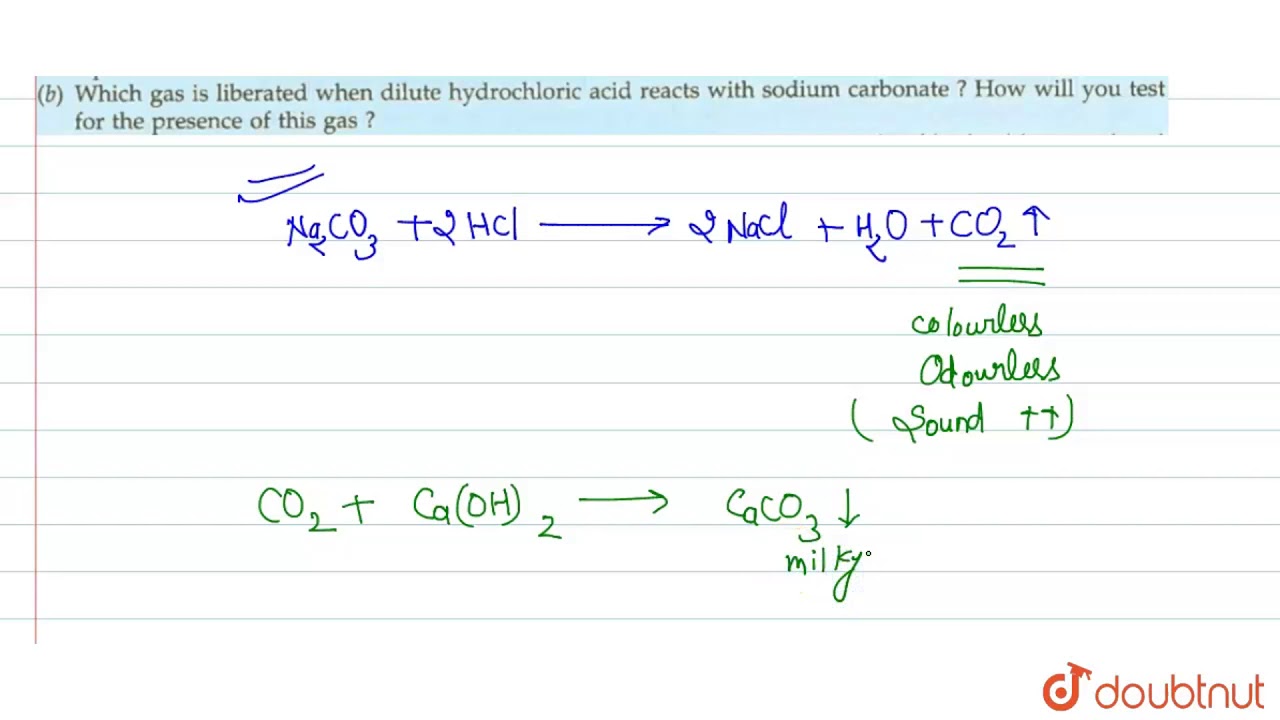
A What Happens When Dilute Hydrochloric Acid Is Added To Sodium Carbonate Write A Balanced Youtube

Four Students Studied Reactions Of Zinc And Sodium Carbonate With Dilute Hydrochloric Acid And Dilute Sodium Hydroxide Solutions And Presented Their Results As Follows The Represents Evolution Of Gas Whereas X
2

How To Balance Nahco3 Hcl Nacl Co2 H2o Sodium Bicarbonate Plus Hydrochloric Acid Youtube
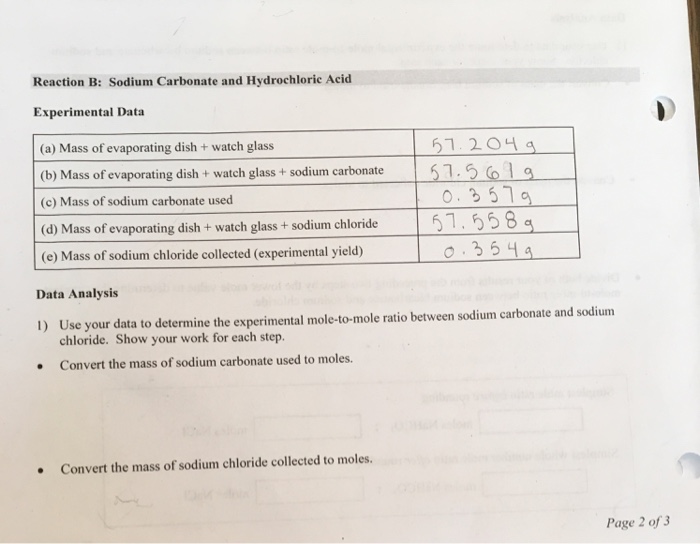
Solved Reaction B Sodium Carbonate And Hydrochloric Acid Chegg Com
0 Response to "Sodium Carbonate And Hydrochloric Acid"
Post a Comment