Polar Vs Nonpolar
In covalent bonding the electrons are shared between the two atomic species involved instead of a complete giveaway or acceptance of electrons. Examples of polar and nonpolar molecules Polar molecules.
9 6 Polar And Non Polar Molecules Physical Science
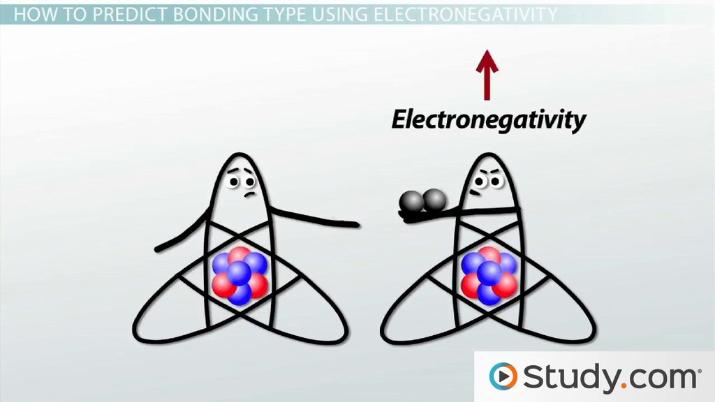
Polar And Nonpolar Covalent Bonds Definitions And Examples Video Lesson Transcript Study Com

Polar Vs Nonpolar Bonds Overview Examples Expii
Polar Protic vs Polar Aprotic vs Nonpolar.
Polar vs nonpolar. One way to remember the difference is to associate the letters of the words with the attribute of the electrons. Polar vs Nonpolar whats the difference and how do I remember which is which. In part c the polar covalent bonds are shown as electron dots shared by the oxygen and hydrogen atoms.
Their R-groups will be pure hydrocarbon alkyl groups alkane branches or aromatic benzene rings. Polar molecules occur when two atoms do not share electrons equally in a covalent bondA dipole forms with part of the molecule carrying a slight positive charge and the other part carrying a slight negative charge. Waters hydrogen bonds create an environment that is.
Intermolecular Forces - Ionic Polar Non-polar Hydrogen Bonding Vision - Application of alkene cistrans isomers. This is a polar. The atoms of covalent materials are bound tightly to each other in stable molecules but those molecules are generally not very strongly attracted to other molecules in the material.
Comparison of Properties of Ionic and Covalent Compounds. Carbonyls - Aldehydes and Ketones. About Solvents In Organic Chemistry A lot of students I talk to have questions about solvents so Ive decided to put together a reference post on them.
Hydrocarbons gasoline toluene homo-nuclear diatomic molecules O 2 N 2 Cl 2 H 2 etc noble gases benzene methane ethylene carbon tetrachloride. Consisting of molecules not having a dipole. A molecule in which the bond dipoles present do not cancel each other out and thus results in a molecular dipolesee below.
Electronegativity values of course. Water is a polar molecule and also acts as a polar solvent. Ionic Bond Covalent Bond James Bond so many bonds.
A polar molecule always contains polar bonds but some molecules with polar bonds are nonpolar. Amino acid are organic compounds. Label the high concentration side and low concentration side.
Polar is a type of covalent bond where atoms share electrons unequally. How to Identify Molecules as Polar or Non-Polar. The positive charge comes from the atomic nucleus while the electrons supply the negative charge.
How to use nonpolar in a sentence. Each diagram shows the unsymmetrical shape of the water molecule. Polar Molecules.
An extreme difference forms an ionic bond while a lesser. Figure PageIndex1 Polar versus Nonpolar Covalent Bonds. Both polar bonds and non-polar bonds are two types of covalent bonding between atoms.
Learn about polar and nonpolar covalent bonds through examples and explore peptide bonds electronegativity and. A compound may possess the polar covalent bonds but it may not be a polar compound. They are described as hydrophobic or water fearing.
Molecules come in infinite varieties. Endocytosis and Exocytosis 5. Nonpolar is a type of covalent bond where atoms share electrons equally.
Atoms have a variety of bonds that affect whether and how they share electrons. Polarity in international relations is any of the various ways in which power is distributed within the international system. The right side after viewing the arrow indicating the direction of movement.
Different ways of representing the polar sharing of electrons in a water molecule. This happens when there is a difference between the electronegativity values of each atom. In a b the polar covalent bonds are shown as lines.
Water alcohol Sulphur dioxide ammonia ethanol hydrogen sulfide bent molecules those with a significant bond angle in general. Differences Between Polar Nonpolar in Chemistry. A molecule may be polar or non-polar.
In general pyramid-shaped and V-shaped molecules are said to be polar. Solvents can cause considerable confusion in reactions because theyre listed along with the reagents of a reaction but often dont actually participate in the reaction itself. The key difference between polar and nonpolar amino acids is that polar amino acids have polarity whereas polarity is absent in nonpolar amino acids.
What dictates which kind of bond will form. Nonpolar molecules do not dissolve easily in water. Polar heads and nonpolar tails.
Because of the nature of ionic and covalent bonds the materials produced by those bonds tend to have quite different macroscopic properties. One generally distinguishes three types of systems. This is a nonpolar covalent bond.
The exception to this is the aromatic amino acid Tyrosine which is polar. The type of system is completely dependent on the. Main Difference Polar vs Nonpolar Bonds.
The reason behind it due to the presence of net dipole in a polar compound they are asymmetrically arrayed. How to Explain Polarity. Some examples of nonpolar molecules are CO 2 H 2 benzene etc.
It describes the nature of the international system at any given period of time. PLEASE WATCH WITH ANNOTATIONS ON. The difference between polar and nonpolar molecules can thus be found by the vectors of partial charge resulting from each bond.
The difference in Electronegativity is the major reason due to the difference between polar and nonpolar bonds. Lets go through each. Polar vs Nonpolar Covalent Bonds As proposed by the American chemist GNLewis atoms are stable when they contain eight electrons in their valence shell.
A The electrons in the covalent bond are equally shared by both hydrogen atoms. 7 Major Differences Polar vs Nonpolar Molecules. An amino acid is composed of.
SOME INACCURACIES IN GRAPHICS ARE NOTED AND CORRECTED IN ANNOTATIONS. B The fluorine atom attracts the electrons in the bond more than the hydrogen atom does leading to an imbalance in the electron distribution. Therefore they are not stable.
Unipolarity bipolarity and multipolarity for three or more centers of power. Natural Biochemical Cycles - carbon nitrogen and phosphorus cycles. A non-polar molecule has a structure of its atoms lined up in a way that the orbital electrons in the outer region cancel out the electronegativity.
When a chemical species is said to be polar this means that the positive and negative electrical charges are unevenly distributed. Amino acids can be divided into two groups based on the polarity as polar amino acids and nonpolar amino acids. Alanine Cysteine Glycine Isoleucine Leucine Methionine Phenylalanine Proline Tryptophan Valine.
Nonpolar molecules do not show any orientation in an electric field as no electrostatic interactions occur between nonpolar molecules and the electric field. Cancellation depends on the shape of the molecule or Stereochemistry and the orientation of the polar bonds. Decide a certain amount to place on the left vs.
When put into polar environments such as water nonpolar molecules stick together and form a tight membrane preventing water from surrounding the molecule. Key Difference Polar vs Nonpolar Amino Acids. Whereas the Linear molecules are said to be non-polar in nature.
The meaning of nonpolar is not polar. Molecular Polarity - Electrostatic Potential Simple Inorganics Organic Functional Groups. Most of the atoms have less than eight electrons in their valence shells except the noble gases in the group 18 of the periodic table.
Polar Vs Nonpolar Bonds And Molecules Schooltube Safe Video Sharing And Management For K12
What Is The Simple Explanation For Polar And Non Polar Substance Quora
Difference Between Polar And Nonpolar Dielectrics Definition Polarity Examples And Differences
Powerschool Learning 8th Grade Science Sec 2 Bond Polarity
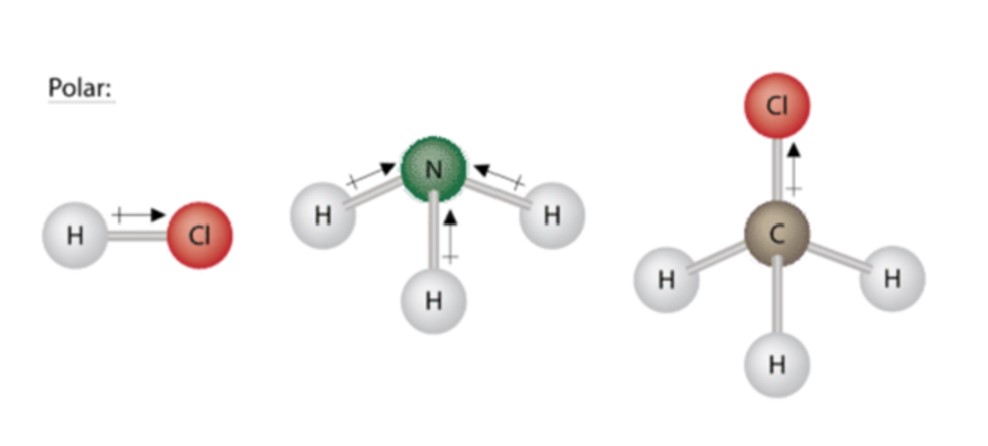
4 12 Shapes And Properties Polar And Nonpolar Molecules Chemistry Libretexts

Polar Vs Non Polar Bonds Molecules Chemtalk
How Does A Polar Covalent Bond Differ From An Nonpolar Socratic

Difference Between Polar And Nonpolar Solvents Compare The Difference Between Similar Terms
0 Response to "Polar Vs Nonpolar"
Post a Comment