Electron Geometry Of Bf3
Double and triple bonds count as ONE REGION OF HIGH ELECTRON DENSITY. The structure formed in the plane suggests that the molecular geometry of BF3 has the shape of trigonal planar central atoms are surrounded by three-terminal atoms.
Solved Indicate The Electron Pair Gcometry And The Molecular Geometry For Each Of The Six Compounds Compound Electron Pair Geometry Molecular Geometry Becl Bf3 802 Ch Socl Of 2

Bf3 Boron Trifluoride Molecular Geometry Bond Angles And Electron Geometry Youtube
According To The Vsepr Theory Which Shape Is Possible For A Molecule With The Molecular Formula Of Ab 3 Where The Number Of Total Electron Groups Is Unstated Socratic
Chemistry 1 Molecular Geometry Review.
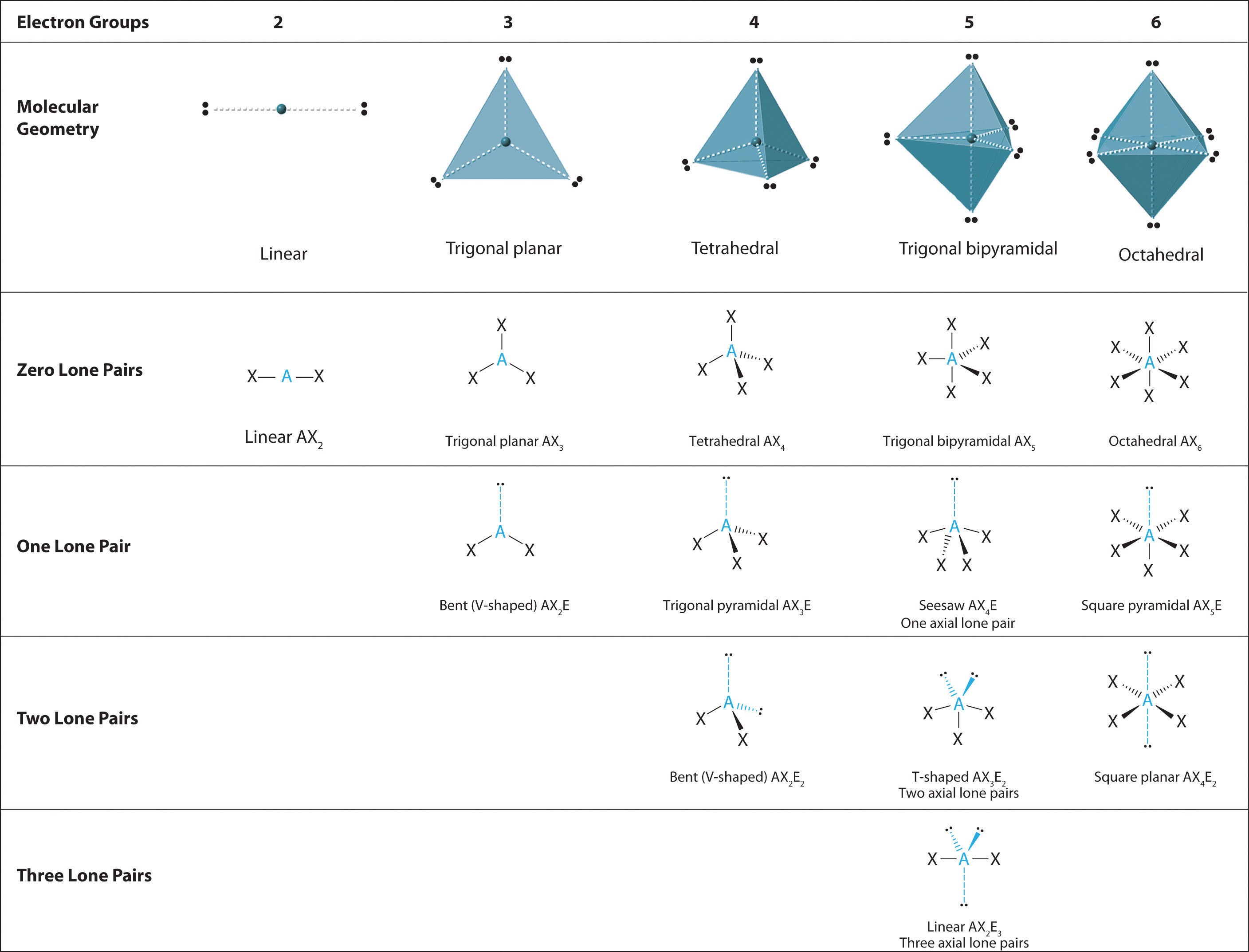
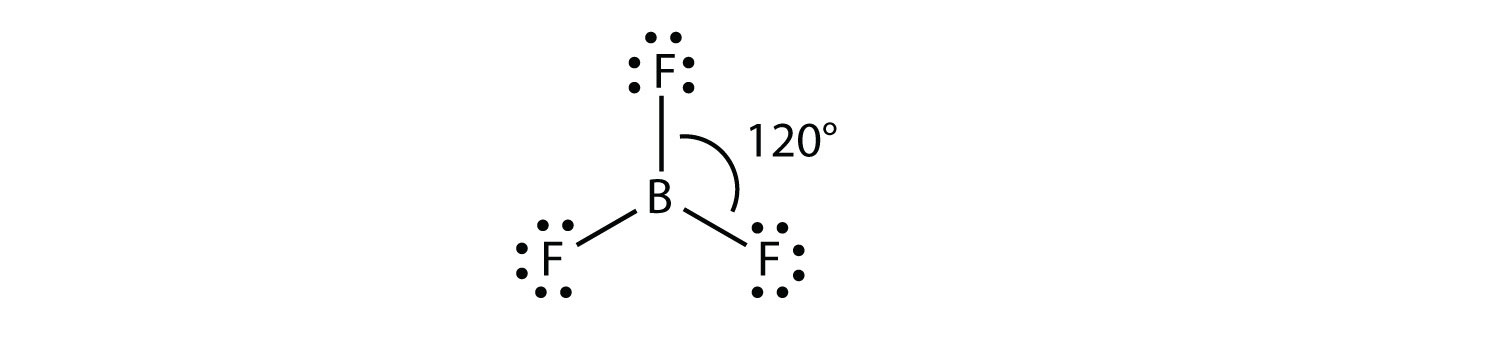
Electron geometry of bf3. This shape makes an equilateral triangle with each side making a 120-degree angle. Its like peripheral atoms all in one plane as all three of them are similar with the 120 bond angles on each that makes them an equilateral triangle. Dec 11 2019 Get the detailed answer.
Lewis electron dot structure calcium hydride. Read More About Hybridization of Other Chemical Compounds. The sulfur core atom two single bonds connected to two.
A a single covalent bond D a total of 8 x 2 16 electron dots. 25 According to VSEPR theory if there are five electron domains in the valence shell of an atom they will be arranged in an _____ geometry. You could think about all this in a plane here.
BF3 violates the octet rule for the central atom. One suggestion is that the F atom is small compared to the larger Cl and Br atoms and the lone pair electron in p z of F is readily and easily donated and overlapped to empty p z orbital of boron. Calculation of formal charge on sulfur atom in SBr2 molecule.
Molecules with less than eight electrons. The F-S-F bonds are slated to be at 90 degrees. A CF4 B NF3 C.
However the hydrogen bond is relatively easy to break when compared with a covalent bond since it has bond energy in the range of only 10 to 30 kilo-joules kJ per mole but large number of The geometry of PCl 5 is trigonal bipyramidal. Actually this entire molecule is planar. Atetrahedral Blinear Coctahedral Dtrigonal planar Etrigonal bipyramidal 26 The electron-domain geometry and molecular geometry of iodine trichloride are _____ and _____ respectively.
The electron dot formula for O2 shows. 3 bonds 0 lone pairs 120 bond angle AB3 type Ex. Terms in this set 13 Linear.
2 bonds 1 lone pair slightly less than 120 bond angle AB2E. An example is the BF3. The geometry of a molecule of BF 3 is trigonal planar.
It comprises one Iodine atom and three Fluorine atoms. What is the Hybridization of Boron Trifluoride. The octet Jun 16 2021 1.
As a result they are nonpolar molecules by nature examples. VE S Valence electron in a sulfur atom of SBr2 molecule. The geometry of molecule of BF3 is Trigonal Planar With the reference of Chemistry Trigonal Planar is a model with three atoms around one atom in the middle.
VSEPR theory predicts that due to the presence of six fluoride ligands and one lone pair of electrons the structure lacks perfect octahedral symmetry and indeed electron diffraction combined with high-level calculations indicate that the Valence shell electron pair repulsion theory or VSEPR theory ˈ v ɛ s p ər v ə ˈ s ɛ p ər VESP-ər. BF 3 has a boron atom with three outer-shell electrons in its ground state and three fluorine atoms containing seven outer electrons. During the formation of this compound the 2s orbital and two 2p orbitals hybridize.
Determine the electron geometry molecular geometry and idealized bond angles for each of the following molecules. A 1 B 2 C 3 D 4 E 5. B a double covalent bond E a total of 32 electron dots.
This model that we use to find out the exact molecular shape of any given composition is a theoretical approach that depends on the repulsive nature of like charges of electron clouds. NH3 BF3 H2O FeCl3 OH - H3O SO3 CCl2. BE Bond pair electron in S atom of SBr2 molecule.
The geometry of the atoms around this carbon happens to be planar. Lewis electron structures give no information about molecular geometry the arrangement of bonded atoms in a molecule or polyatomic ion which is crucial to understanding the chemistry of a molecule. C an ionic bond Ans.
Molecular geometry is an essential concept based on VSEPR theory that helps us determine the 3D structure of a molecule. CH3 - C O 7. Hybridization of the central atom d.
Consider the following electronegativity values. H 21 Cl 30 F 40. BF3 has a trigonal planar molecular geometry.
Electron and Molecular Geometries. 2 Bonds 0 Lone Pairs 180 degree bond angle AB2 Type Ex. Were thinking about its chemical behavior one of the things that BF3 can do the Boron can accept an electron pair and function as a lewis acid.
The boron in BF3 is electron poor and has an empty orbital so it can accept a pair of electrons making it a Lewis acid. Vsepr lab answer key. Give the electron geometry eg molecular geometry mg and hybridization for XeF4.
The valence-shell electron-pair repulsion VSEPR model allows us to predict which of the possible structures is actually observed in most cases. Dipole moment View Answer Draw the Lewis structure for XeF4 and provide the following information. VSEPR theory stands for Valence Shell Electron Pair Repulsion Theory.
Each orbital gets a single electron in an sp2 loop. Click hereto get an answer to your question Categorise the following moleculesions as nucleophile or electrophile1. There are molecules with a polar bond but the molecular geometry is symmetrical.
SF6 molecular geometry will be octahedral because if we look at the structure sulphur hexafluoride has a central sulphur atom around which12 electrons or 6 electron pairs are present and there are no lone pairs. Further if we observe closely one boron electron is unpaired in the ground state. Sf4 2 lewis structure email protected.
LES Lone pairs of an electron in the sulfur atom of the SBr2 molecule. Click hereto get an answer to your question Classify the following into electrophiles and nucleophiles. The number of lone electron pairs in the N2 molecule is ___.
The electron geometry and the molecular geometry of ammonia NH3 are respectively. 410 və-SEP-ər is a model used in.

Icl3 Molecular Geometry Science Education And Tutorials
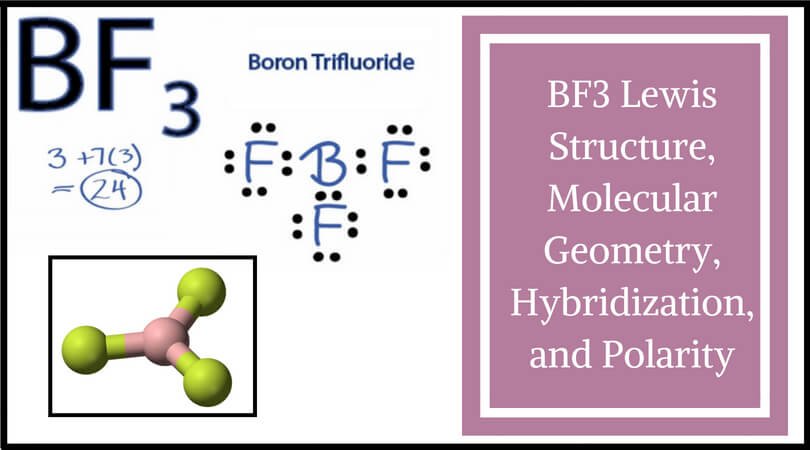
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity
Molecular Shapes

Ppt What Is The Shape Of Bf 3 You Do Not Need To Take Notes This Is Just A Review On What We Did Before Our Break Ju Powerpoint Presentation Id 562284

Valence Shell Electron Pair Repulsion Theory Vsepr
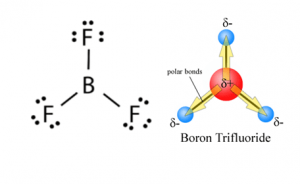
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity

Molecular Geometry And Covalent Bonding Models
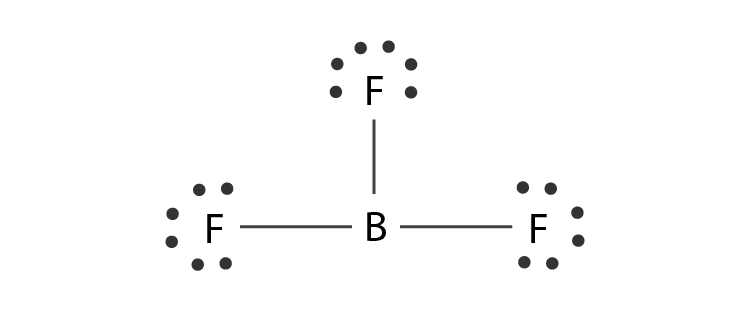
Hybridization Of Bf3 Hybridization Of Boron Fluoride In Bf3
0 Response to "Electron Geometry Of Bf3"
Post a Comment