Addition Reactions Of Alkenes
We will review their nomenclature and also learn about the vast possibility of reactions using alkenes and alkynes as starting materials. The electrophilic addition of bromine to ethene.
2
Introduction To Addition Reactions Master Organic Chemistry

The Characteristic Reaction Of Alkenes Is Addition To The Double Bond A B C C A C C B Reactions Of Alkenes Ppt Download
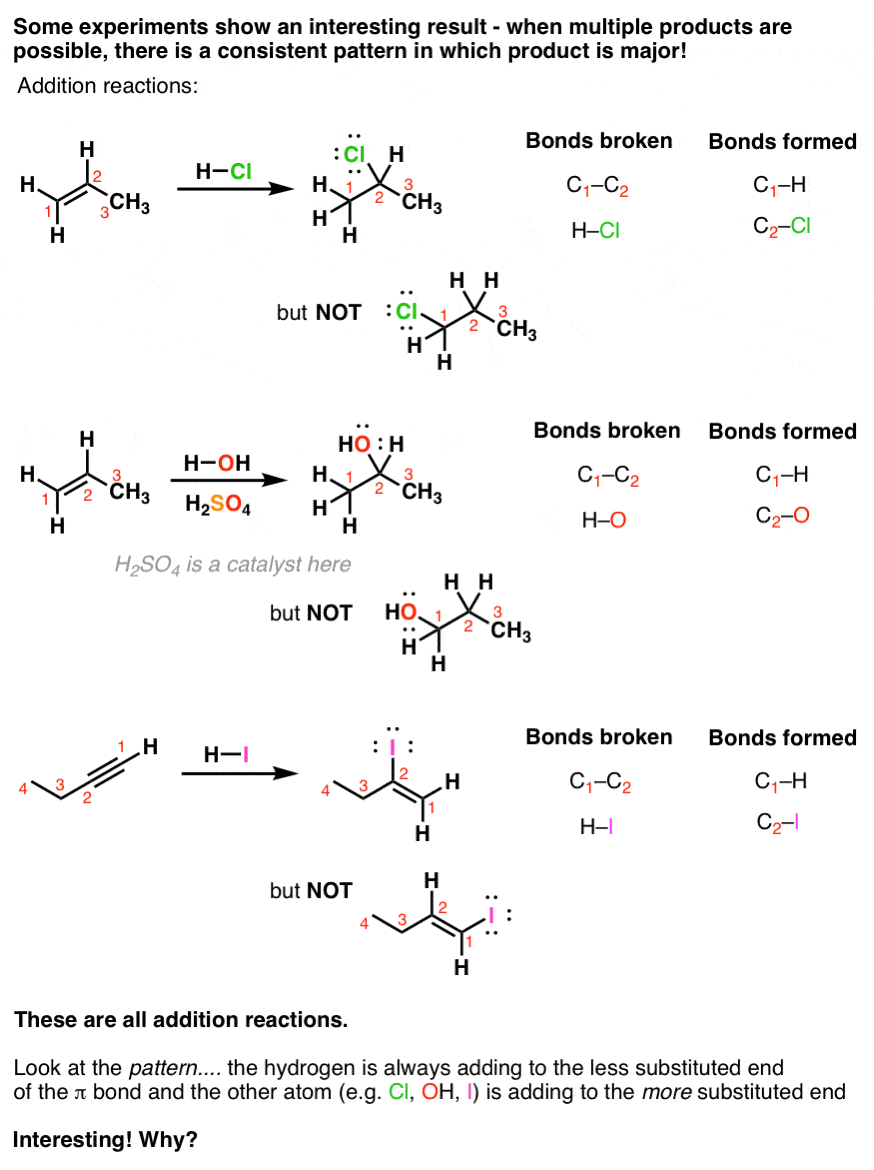
Addition of a H-B bond to C-C double bonds.
Addition reactions of alkenes. Cycloalkenes are named the same way that open chain chained alkenes are with the exception that the numbering is started at one of the carbons of the double bond in order to keep the index numbers as small as possible. Remember Markovnikovs rule in the addition of electrophiles to alkenes. Other types of reaction have been substitution and elimination.
Organic Reactions provides a compilation of an authoritative summary of a preparatively useful organic reaction from the primary literature. The double bond CC is the active site on the molecule. Hydrogenation is a reaction in which hydrogen H 2 adds across the double bond.
Watch the video below on alkenes and cycloalkenes. Alkenes are unsaturated hydrocarbons. This video covers the two common cyclopropanation reactions the haloform and Simmons-Smith reactions.
Hydrogenation of alkenes is a reduction reaction. Addition reactions involving alkenes and alkynes include hydrogenation halogenation and hydrohalogenation. Helical peptide foldamers catalyze Michael addition reactions of nitroalkanes or dialkyl malonates to αβ-unsaturated ketones to give Michael adducts with high enantioselectivities.
Addition reactions are typically exothermic. 46 Addition elimination and substitution reactions ESCKY Mechanisms and arrow-pushing are not required by CAPS. For example take an alkene like 1-butene and add HBr.
2021 86 5292Bill Morandi of ETH Zürich effected the selective azido amination of the alkene 3 leading to 4 J. Waaay back in the day we talked about addition reactions of alkenes. Examples are hydrohalogenation halogenation halohydrin formation oxymercuration hydroboration dichlorocarbene addition SimmonsSmith reaction catalytic hydrogenation epoxidation radical polymerization and.
Video 14 Cyclopropanation of Alkenes. Syn vs Anti In the last post on alkene addition reactions we discussed one of the two key themes to look for in addition reactions. Mechanism Explanation 448 Polymerization of Alkenes 647 Syn Anti Addition in Stereochemistry.
Hydrogenation of alkenes requires a catalyst so it is also referred to as catalytic hydrogenation. These addition reactions are analogous to those of the alkenes. The addition of hydrogen to compounds happens in a syn addition fashion adding to the same face of the compound and entering from the least hindered side.
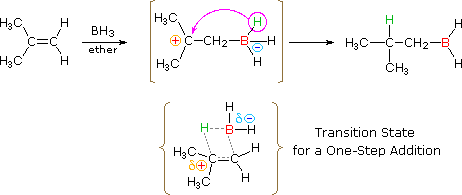
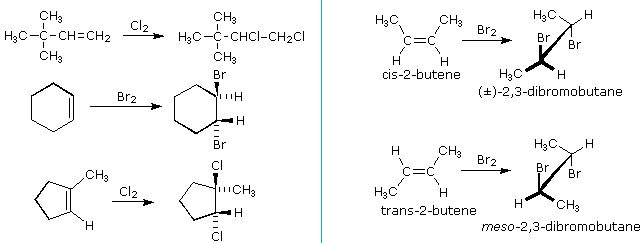
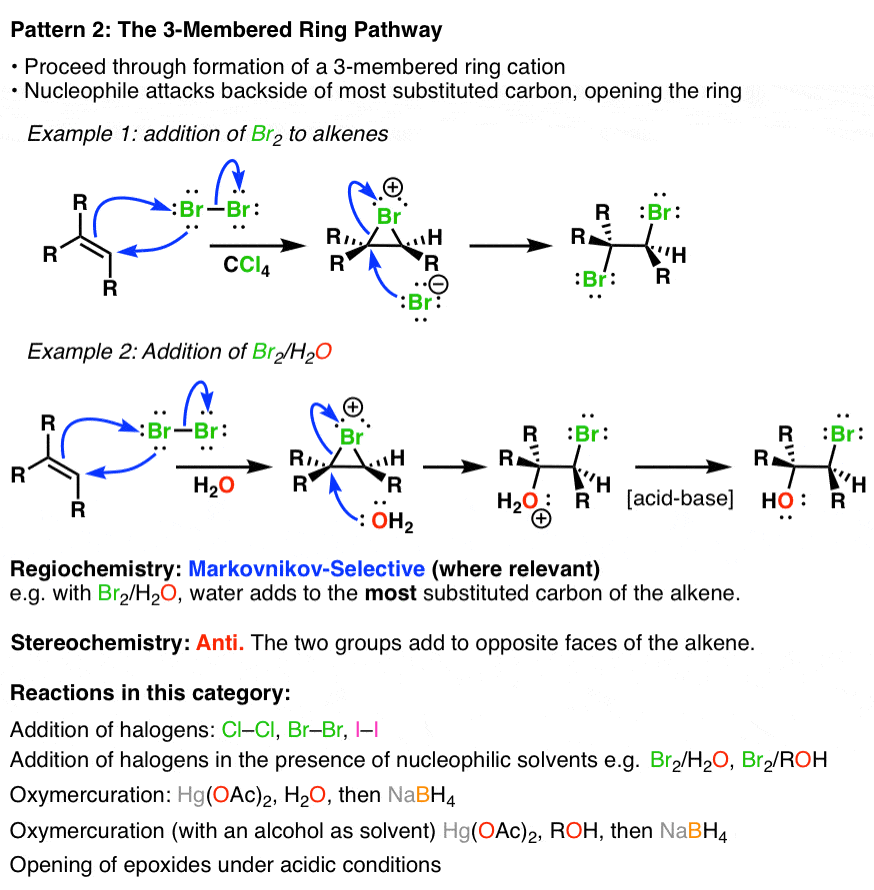
Catalytic Hydrogenation of Alkenes. This page gives you the facts and a simple uncluttered mechanism for the electrophilic addition reactions between bromine and the other halogens and alkenes like ethene and cyclohexene. Hydroboration proceeds via a four-membered transition state.
Electrophilic addition of bromine to cyclohexene. The reaction of one equivalent of bromine with 1-penten-4-yne for example gave 45-dibromo-1-pentyne as the chief product. A hydrogen atom joins to one of the carbon atoms originally in the double bond and a halogen atom to the other.
Hydrogenation reactions typically employ a metallic catalyst consisting of platinum nickel palladium or rhodium. In both an alkene reacts through a concerted reaction mechanism to form a cyclopropane in place of its pi bond. The amide protons at the N terminus in the α-helical peptide catalyst are crucial for activating Michael donors while the N-terminal primary amine activates Michael acceptors through the formation of iminium.
35 Which of the following is the best reaction sequence to accomplish a Markovnikov addition of water to. The most common and thermodynamically favored chemical transformations of alkenes are addition reactions. Alkenes and alkynes are useful reagents in polymer synthesisan important industrial application.
The hydrogen and the boron atoms added on the same face of the. Many of these addition reactions are known to proceed in a stepwise fashion by way of reactive intermediates and this is the mechanism followed by most polymerizations. Addition To Alkenes Revisited.
Practitioners interested in executing such a reaction or simply learning about the features advantages and limitations of this process thus have a valuable resource to guide their experimentation. Platinum palladium nickel and. Generally alkenes will convert to alkanes alkynes to alkenes aldehydes and ketones to alcohols esters to secondary alcohols and amides to amines via hydrogenation reactions.
The Park Synthesis of Nitramine. Skip to 602 for an example on naming a cycloalkene. Hydroboration is typically anti-Markovnikov ie.
Mechanism Reactions Examples 422. The most common chemical transformation of a carbon-carbon double bond is the addition reactionA large number of reagents both inorganic and organic have been found to add to this functional group and in this section we shall review many of these reactions. All alkenes undergo addition reactions with the hydrogen halides.
Alkynes undergo catalytic hydrogenation with the same catalysts used in alkene hydrogenation. For example with ethene and hydrogen chloride you get chloroethane. Regiochemistry in other words what is the favored direction in which the pi-bond breaks.
Nucleophilic Reactions of Acetylenic Anions Terminal alkynes. Gong-Qing Liu and Yong Ling of Nantong University prepared the selenyl alcohol 2 by the oxidative addition of diphenyl diselenide to the alkene 1 J. The learners need to know the types of reactants the types of reactions and the reaction conditions.
Alkenes react in many addition reactions which occur by opening up the double-bond. With but-2-ene you get 2-chlorobutane. Like in the case of alkenes the catalytic addition of hydrogen to alkynes is a syn process giving a cis-product.
The hydrogen adds to the most substituted carbon of the double bondThat the regiochemistry is reverse of a typical HX addition reflects the polarity of the B δ-H δ bonds. Stereoselectivity In Alkene Addition Reactions. Ch08 Reacns of Alkenes landscape Page 1 Reactions of Alkenes Since bonds are stronger than bonds double bonds tend to react to convert the double bond into bonds This is an addition reaction.
A nucleophilic addition reaction is an addition reaction where a chemical compound with an electron-deficient or electrophilic double or triple bond a π bond reacts with a nucleophile which is an electron-rich reactant with the disappearance of the double bond and. Addition Reactions of Alkenes. This illustrates the principle of _____.
34 The mechanism for the acid-catalyzed hydration of alkenes is the reverse of the acid-catalyzed dehydration of alcohols. We will study three main types of reactions - addition elimination and substitution. Most of these addition reactions follow the mechanism of electrophilic addition.
This post is about the second key theme in addition reactions of alkenes. Alkenes and alkynes can be transformed into almost any other functional group you can name. The reactions are even more exothermic than the additions to alkenes and yet the rate of addition to alkynes is slower by a factor of 100 to 1000 than addition to equivalently substituted alkenes.

Organic Chemistry Addition Reactions Of Alkenes O Level Secondary Chemistry Tuition
Alkene Reactivity
Summary Three Key Families Of Alkene Reaction Mechanisms

Alkene Reactivity

Alkene Addition Reaction Ppt Download
Chapter 5 Addition Reactions Of Alkenes
Lesson Explainer Addition Reactions Of Alkenes Nagwa
Addition Reaction Of Alkenes With Hydrogen Easy Exam Revision Notes For Gsce Chemistry

0 Response to "Addition Reactions Of Alkenes"
Post a Comment