Reactivity Series Of Metals
The Reactivity Series of Metals Towards Oxygen The reactivity of metals differs from one metal to another. So reactivity series of metals can be defined as a series of metals in order of reactivity from highest to lowest.


Reactivity Series Investigation Instruction Sheet Print Out

5 4 2 Reactivity Series And Extraction Of Metals Spm Science Chemistry Basics Study Chemistry Teaching Chemistry
The arrangement of metals in a vertical column in order of their decreasing reactivity downwards is called the reactivity series of metals.

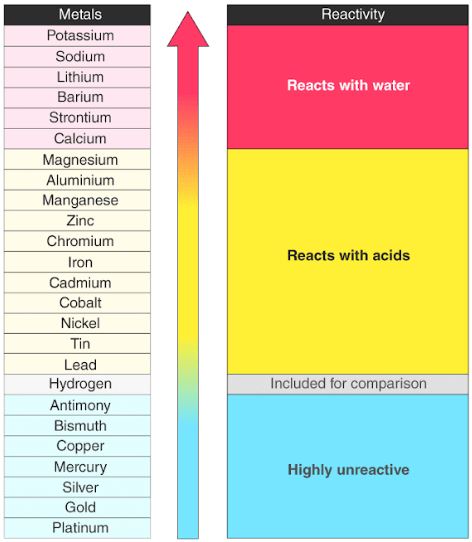
Reactivity series of metals. The reactivity of different metals with oxygen is different - Metals like potassium and sodium react vigorously with oxygen and catch fire if kept in open. Gold has very low reactivity and therefore can be found in its metallic state in nature. It is used to summarize information about the reactions of metals with acids and water single displacement reactions and the extraction of metals from their ores.
In fact the form in which a metal occurs in nature depends on its reactivity. CuCuSO 4 reference electrodes. Metals and Non-metals Class 10 Important Questions Long Answer Type.
The reactivity series allows us to predict how metals will react. Zn - Zinc Reactivity decreases. This is due to the build-up of electrons in the immediately underlying d-sub-shells that efficiently shields the 4s electrons from the nucleus and minimizing the increase in effective nuclear charge Z_eff from element to element.
K - Potassium Most reactive. The reactivity series of metals can be shown in another way which includes oxidation reaction of each metal to the respective metal ion. The lanthanides are commonly referred to as the rare earth elements REE although many people group.
This experiment will involve studying the reactivity of select metals Ca Cu Fe Mg Sn and Zn in order to generate an activity series for these elements. Na - Sodium. In addition you will investigate the reactivity of a variety of different metals to generate an activity series for these elements.
It is also known as activity series. Also after performing the experiment Reactivity Series of Metals you will be able to analyse the decreasing order of their reactivity. Hence they are stored in kerosene to prevent burning.
A metal can displace a less reactive metal from its compounds. Observing the action of zinc iron copper and aluminium metals for the following salt solutions. Reactivity Series of Metals.
Rusting is an oxidation reaction. It can be used to predict the products in similar reactions involving a different metal. Metals can be arranged on the basis of relative reactivity.
These metals are known as Noble metals. Non-metals form acidic oxides with oxygen of air. 62 Recall that alkali metals.
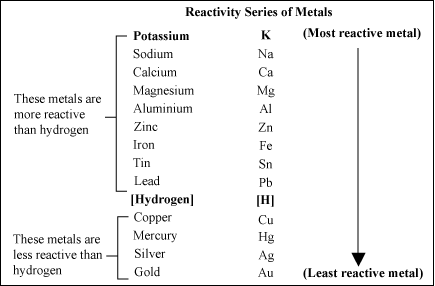
Topic 6 - Groups in the periodic table. These trends can also be used to identify an unknown element. Hydrogen - hydrogen - Reactivity of hydrogen.
B i The blue solution will become colourless and reddish brown copper metal will be deposited. A spectrochemical series is a list of ligands ordered by ligand strength and a list of metal ions based on oxidation number group and elementFor a metal ion the ligands modify the difference in energy Δ between the d orbitals called the ligand-field splitting parameter in ligand field theory or the crystal-field splitting parameter in crystal field theory. Reactivity series of metals.
In each Part the observed trends will be compared with the arrangement of the elements in the periodic table. The lanthanides or lanthanoid series is a group of transition metals located on the periodic table in the first row period below the main body of the table. The dissociation energy of molecular hydrogen is 104000 calories per molewritten 104 kcalmole mole.
The below diagram lists metals and alloys potential in the order of reactivity in sea water vs. In the reactivity series copper gold and silver are at the bottom and hence least reactive. Non-metals cannot displace hydrogen from dilute acids.
This is useful for extracting metals from their oxides. Reactivity series is the series of metals based on their reactivity from highest to lowest. Galvanic series diagram.
A The series of metals in which metals are arranged in decreasing order of their reactivity. In moving across the series of metals from scandium to zinc a small change in the values of the first and second ionization energies is observed. Top listed metalsalloys are the least active most noble.
In Group 1 the reactivity of the elements increases going down the group. A more reactive metal will displace a less reactive metal from a compound. In reactivity series the most reactive metal K is placed at the top of the series while the least reactive metal Au is placed at the bottom.
One molecule of hydrogen dissociates into two atoms H2 2H when an energy equal to or greater than the dissociation energy ie the amount of energy required to break the bond that holds together the atoms in the molecule is supplied. Reactivity of elements decreases on moving from top to bottom in the given reactivity series. The reactivity series of metals also known as the activity series refers to the arrangement of metals in the descending order of their reactivities.
In chemistry a reactivity series or activity series is an empirical calculated and structurally analytical progression of a series of metals arranged by their reactivity from highest to lowest. 63 Describe the reactions of lithium sodium and potassium with water. The activity series of metals is an empirical tool used to predict products in displacement reactions and reactivity of metals with water and acids in replacement reactions and ore extraction.
It gives information regarding the reducing power of the metal atom and the oxidation number of the metal ion. Are soft have relatively low melting points. The alkaline earths Group 2 and the halogens Group 17.
Topic 6 - Groups in the. Aluminium potassium and sodium have very. Summary notes flashcards and past exam questions by topic for CAIE IGCSE Chemistry Topic 10 - Metals.
Zn and AgAgCl and in soil vs. Au Cu Fe Mg is increasing order of reactivity. 43 Explain the reactivity series of metals potassium sodium calcium magnesium aluminium carbon zinc iron hydrogen copper silver gold in terms of the reactivity of the metals with water and dilute acids and that these reactions show the 45 Explain oxidation as the gain of oxygen and reduction as the loss of oxygen.
The order of intensity or reactivity of metal is known as Reactivity Series. The data provided by the reactivity series can be used to predict whether a metal can displace another in a single displacement reaction. Zinc sulphate ZnSO 4 Copper sulphate CuSO 4 Ferrous sulphate FeSO 4 Aluminium sulphate Al 2 SO 4 3.
The reactivity series lists metals from the most reactive to the least reactive. Plore these trends in reactivity for the elements for two different groups. The arranging of metals in the decreasing order of their reactivity is called reactivity series of metals.
Metals show displacement reactions on the basis of their reactivity series. Non-metals do not show such displacement reactions.

Metals Learning Objectives Use Reactivity Data To Determine A Reactivity Series Relate Extraction Method To Reactivity Of Metals Write Word Symbol Equations Ppt Download

Metals And Non Metals Reactivity Series Easy Trick Chapter 3 Ncert Cbse Youtube
Reactivity Series Reactivity Of Metals Chart Features Uses

Reactivity Series Of Metals O Level Chemistry Notes
What Is An Easy Way To Remember The Metal Reactivity Series Quora
Reactivity Series Teaching Resources
How Does The Metal Reactivity Series Work Example
The Reactivity Series Shows Metals Chemistry Pathshala Facebook




0 Response to "Reactivity Series Of Metals"
Post a Comment