Cubic Unit Cell
Some metals crystallize in an arrangement that has a cubic unit cell with atoms at all of the corners and an atom in the center as shown in Figure 2. For the sake of argument well define the a axis as the vertical axis of our coordinate system as shown in the figure.

Unit Cell Lattice Parameters Cubic Structures Video Lesson Transcript Study Com
Facecentered Cubic Unit Cell Crystal Lattice Stock Illustration 347363273

What Is A Unit Cell Definition Types Of Unit Cell Primitive Unit Cell Bcc Fcc
NaCl has a cubic unit cell.

Cubic unit cell. The face atoms are shared with an adjacent unit cell so each unit cell contains ½ a face atom. Because we know that we will need to move in the negative y-direction let s locate the origin at 0 1 0. However when dealing with mathematical descriptions of crystals it may be easier to describe the unit cell in the smallest form possible.
A third common packing arrangement in metals the body-centered cubic BCC unit cell has atoms at each of the eight corners of a cube plus one atom in the center of the cube. Atoms of the face centered cubic fcc unit cell touch across the face diagonal Figure 9. So the of atoms per cell for BCC is 8x18 12 atoms per unit cell for BCC HCP 12 corner atoms shared by six unit cells each two center face atoms shared by two cells and three atoms fully contained by the unit cell.
The lattice points in a cubic unit cell can be described in terms of a three-dimensional graph. The volume of the cubic unit cell a 3 2r 3 8r 3. Therefore a particular unit cell has the only 18 th of an atom.
A body-centered cubic unit cell has six octahedral voids located at the center of each face of the unit cell and twelve further ones located at the midpoint of each edge of the same cell for a total of six net octahedral voids. So total atoms in the body-centred unit cell will be. The cubic unit cell centred on the body is the simplest repeating unit in a cubic structure centred on the body.
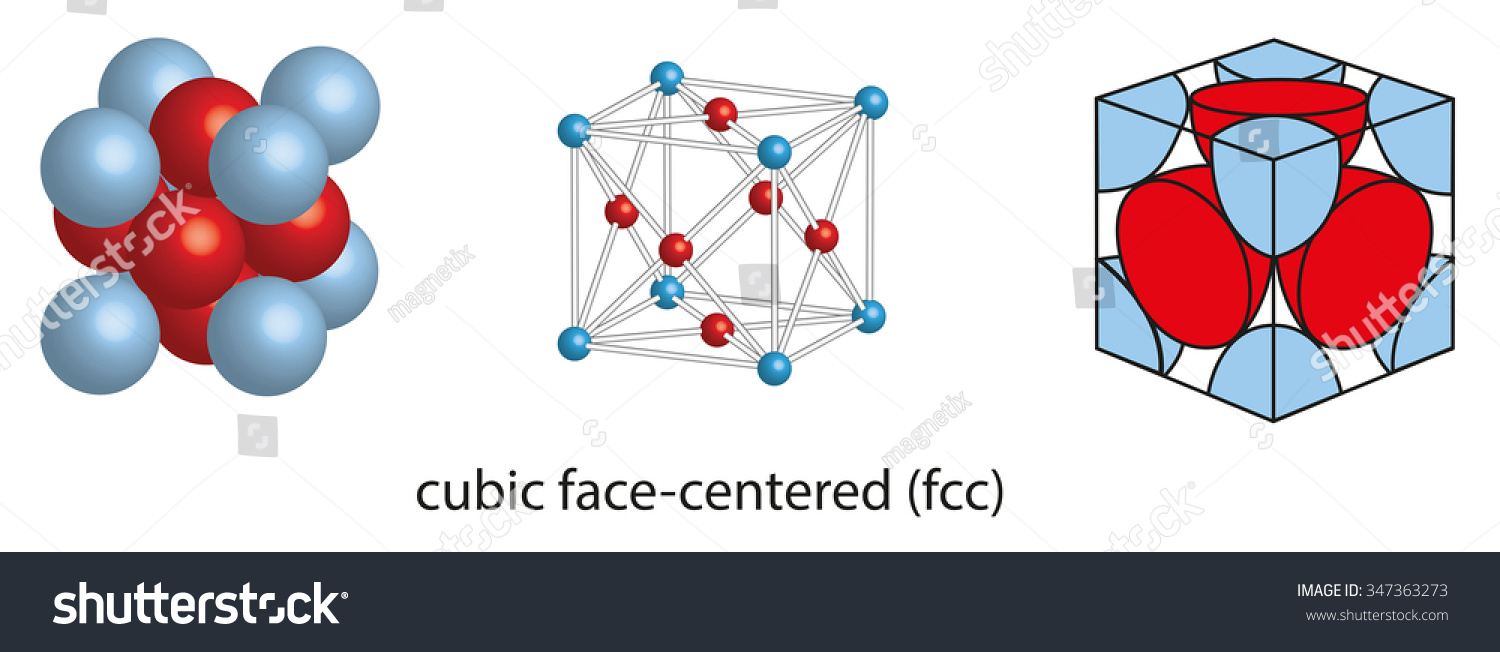
The fcc unit cell contains 8 corner atoms and an atom in each face. In the structure drawn all of the particles yellow are the same. Each ion is 6-coordinate and has a local octahedral geometry.
Thus 476 volume is empty space void space ie. Reason Besides the body center there is one octahedral void present at the center of each of the six faces of the unit cell and. Additionally there are 24 tetrahedral voids located in a square spacing around each octahedral void.
A 301 and 212. Image Will be Uploaded Soon In this unit cell the atoms in the middle layer are not shared with any other unit cell while the atoms in upper and bottom layers are shared with adjacent unit cells. Many metals pack in cubic unit cells.
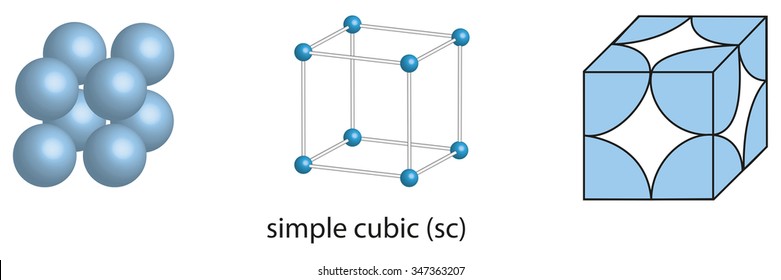
A 2 2 r 3 4 r 2 r. Because all three cell-edge lengths are the same in a cubic unit cell it doesnt matter what orientation is used for the a b and c axes. This unit cell is the simplest for people to understand although it rarely occurs in nature due to its low packing.
A Three-Dimensional Graph. The edge lengths of the unit cell in terms of the radius of spheres constituting fcc bcc and simple cubic unit cell are respectively. Total number of octahedral voids present in unit cell of cubic close packing including the one that is present at the body centre is four.
Almost half the space is empty. Since a simple cubic unit cell contains only 1 atom. B 101 and 313.
Body- centred Cubic Unit Cell. In the primitive cubic unit cell the atoms are present only at the corners. Sketch and label the following directions and planes in cubic unit cells one sketch per cell.
121 210 Construction of a a direction and b plane within a unit cell. The tail of the direction will be located at this new. The unit cell is the smallest part of a crystal that repeated regularly through translation in three dimensions creates the whole crystal.
SC has 1 atom per unit cell lattice constant a 2r Coordination Number CN. Because each of the corner atoms is the corner of another cube the corner atoms in. It is best thought of as a face-centered cubic array of anions with an interpenetrating fcc cation lattice or vice-versa.
The atom at the corners of the cube are shared with eight other unit cells. The central sphere or atom in top and bottom layers is shared with 1 other unit cell hence they only. Since 8 atoms are present at the corners each will contribute 18 th of the original volume of the cell.
Primitive Cubic Unit Cell. The density of a metal and length of the unit cell can be used to determine the type for packing. For example sodium has a density of 0968 gcm 3 and a unit cell side length a of 429 Å a.
This conventional cell has advantages because it is highly symmetric and easy for humans to understand. In a body-centred unit cell 8 atoms are located on the 8 corners and 1 atom is present at the center of the structure. Every atom at the corner is shared among 8 adjacent unit cells.
Once again the eight corners of the unit cell contain eight similar particles. For example the image shown here is the unit cell of a primitive cubic structure. Silicon crystallizes in the same pattern as diamond in a structure which Ashcroft and Mermin call two interpenetrating face-centered cubic primitive lattices.
Unit cell corner is described by a lattice point at which the crystal contains an atom ion or molecule. Thus 12 x 16 2 x 12 3 6 atoms. The unit cell of a crystal is defined by the lattice points.
Body-centered cubic BCC is the name given to a type of atom arrangement found in nature. A simple cubic unit cell has a single cubic void in the center. A primitive cell is a unit cell that contains exactly one lattice point.
Cubic definition having three dimensions. Cubic unit cells of metals show in the upper figures the locations of lattice points and in the lower figures metal atoms located in the unit cell. The above illustration shows the arrangement of the silicon atoms in a unit cell with the numbers indicating the height of the atom above the base of the cube as a fraction of the cell dimension.
The cell looks the same whether you start with anions or cations on the corners. The diamond cubic cell that I have shown you is a conventional unit cell not a primitive unit cell. There are 4 unit cells in the same layer and 4 in the upper or lower layer.
The packing efficiency of the simple cubic cell is 524. A body-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube shares an atom and with one atom positioned at the center. This problem has been solved.
Hcp unit cell can be represented as. For unit cells generally lattice points that are shared by n cells are counted as 1 n of the lattice points contained in each of those cells. The simple cubic SC unit cell can be imagined as a cube with an atom on each corner.
HCP STRUCTURE ideal ratio ca of 83 1633 unit cell is a simple hexagonal lattice with a two-point basis 000 231312 a a Plan view 0002 planes are close packed ranks in importance with FCC and BCC Bravais lattices 72. So for example a primitive unit cell in three dimensions which has lattice points only at its eight vertices is considered to contain 1 8 of each of them.

Cubic Unit Cells In Crystals Showing Two Specific Arrangements Of Download Scientific Diagram

Within A Cubic Unit Cell Sketch The Following Directions A 101 B 211 C 10 Bar 2 D 3 Bar 1 2 E 3 Bar 1 3 Study Com

Unit Cells Chemistry For Non Majors
Unit Cell Images Stock Photos Vectors Shutterstock

Cubic Unit Cells Study Page

What Is A Simple Cubic Unit Cell Quora

Number Of Atoms Per Unit Cell In A Cubic Unit Cell W3spoint
Face Centered Cubic Fcc Unit Cell Materials Science Engineering
0 Response to "Cubic Unit Cell"
Post a Comment