Bohr Model Of Hydrogen Atom
Bohr Model of the hydrogen atom first proposed the planetary model but later an assumption concerning the electrons was made. He postulated that the electron was restricted to certain orbits characterized by discrete energies.
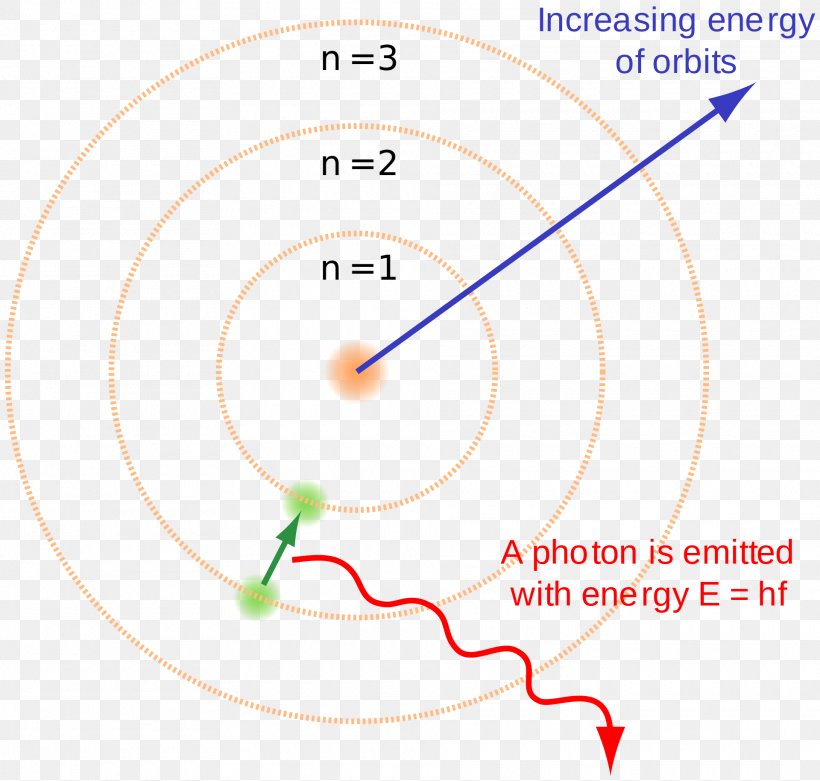
Bohr Model Hydrogen Atom Atomic Theory Energy Level Png 1920x1829px Bohr Model Area Atom Atomic Orbital
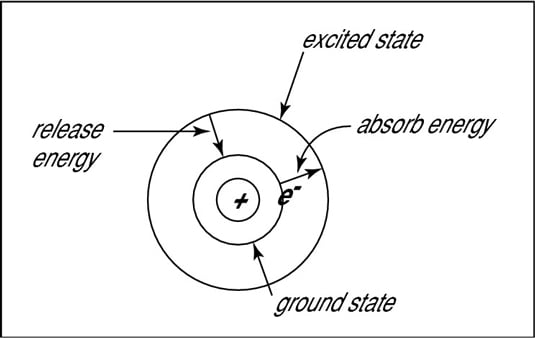
Atomic Structure The Bohr Model Dummies
Historically Bohrs model of the hydrogen atom is the very first model of atomic structure that correctly explained the radiation spectra of atomic hydrogen.
Bohr model of hydrogen atom. Bohr atomic model of a nitrogen atom. Bohr model for nitrogen by Allen Saunders October 18 This is a collection of diagrams of atoms showing the numbers of protons neutrons This diagram shows the electron shell of a nitrogen atom. The Bohr model of the hydrogen atom explains the connection between the quantization of photons and the quantized emission from atoms.
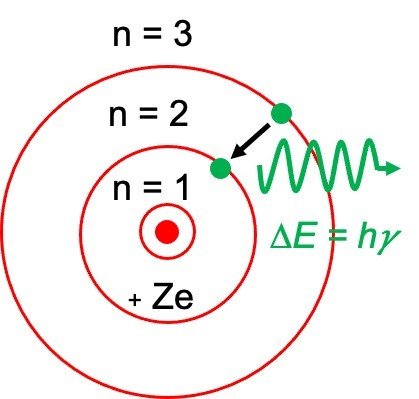
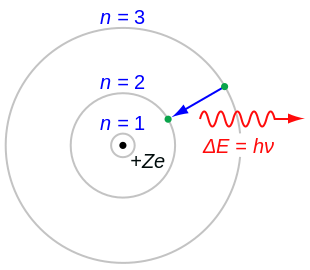
Electron Transitions The Bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy. Check how the prediction of the model matches the experimental results. Bohrs model of hydrogen atom.
He defines the different isotopes of hydrogen. Ernest Rutherfords nuclear model. This lecture includes the following.
The Bohr model is easy to picture but we now know that it is wrong. Bohr described the hydrogen atom in terms of an electron moving in a circular orbit about a nucleus. In 1913 a Danish physicist Niels Bohr 18851962.
Bohr atomic model description of the structure of atoms especially that of hydrogen proposed. The motion of the electrons in the Rutherford model was unstable because according to classical mechanics and electromagnetic theory any charged particle moving on a curved path emits electromagnetic radiation. Electrons can only be in certain discrete circular orbits or stationary states thereby having a discrete set of possible radii and energies.
How Bohrs model of hydrogen explains atomic emission spectra. How did scientists figure out the structure of atoms without looking at them. Although revolutionary at the time the Bohr model is a relatively primitive model of the hydrogen atom compared to the valence shell atom.
This is the currently selected item. Bohr model radii derivation using physics Bohr model radii. Bohrs model of hydrogen.
As a theory it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and. Unfortunately Bohrs model worked only for hydrogen and hydrogenic atoms such as any atom with all but one electron removed. It came into existence with the modification of Rutherfords model of an atom.
In it most of the atoms mass is concentrated into the center what we now call the nucleus and electrons surround the positive mass in something like a cloud. As an initial. Electrons move around a nucleus but only in prescribed orbits and If electrons jump to a lower-energy orbit the difference is sent out as radiation.
Bohrs model of hydrogen. Begingroup Bohr model is ad hoc -type one trick pony for explaining energy levels of hydrogen atom. The simplest example of the Bohr Model is for the hydrogen atom Z 1 or for a hydrogen-like ion Z 1 in which a negatively charged electron orbits a small positively charged nucleus.
The Bohr model is a relatively primitive model of the hydrogen atom compared to the valence shell atom model. According to the Bohr model the wavelength of the light emitted by a hydrogen atom when the electron falls from a high energy n 4 orbit into a lower energy n 2 orbitSubstituting the appropriate values of R H n 1 and n 2 into the equation shown above gives the following result. Whereas in the modern view atomic electron structure is more like 3D standing waves.
The assumption was the quantization of the structure of atoms. Bohr model description of the structure of atoms especially that of hydrogen proposed 1913 by the Danish physicist Niels BohrThe Bohr model of the atom a radical departure from earlier classical descriptions was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. Niels Bohr Danish physicist used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom.
He details Bohrs postulates for the hydrogen atom and discusses how the Planck-Einstein relationship applies to electron transitions. Bohrs proposed that electrons orbited the nucleus in specific orbits or shells with a fixed radius. The main problem with Bohrs model is that it works very well for atoms with only one electron like H or He but not at all for multi-electron atoms.
Emission spectrum of hydrogen. In 1913 Bohr proposed his quantized shell model of the atom to explain how electrons can have stable orbits around the nucleus. Google Classroom Facebook Twitter.
His many contributions to the development of atomic physics and quantum mechanics his personal influence on many students and colleagues and his personal integrity especially in the face of Nazi oppression earned him a prominent place in history. Bohr model radii derivation using physics. Not only did the Bohr model explain the reason for the structure of the Rydberg formula it also provided a justification for its empirical results in terms of fundamental physical constants.
Solving for the wavelength of this light gives a value of 4863 nm which agrees with the experimental. The electron moves around the nucleus in circular orbits that can have only certain allowed radii. Any planetary model of the atom so often seen in pictures and so easy to picture is wrong.
Bohr built upon Rutherfords model of the atom. Nobel Prize in Physics 1922 proposed a theoretical model for the hydrogen atom that explained its emission spectrum. In 1913 Niels Bohr proposed a theory for the hydrogen atom based on quantum theory that some physical quantities only take discrete values.
In 1913 Neils Bohr built on the work of Max Planck and Albert Einstein and proposed that the movement of electrons within an atom was quantized. Science Class 11 Chemistry India Structure of atom Bohrs model of hydrogen atom. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.
Thomsons plum pudding model. The Bohr model and all of its successors describe the properties of. Rutherfords model introduced the nuclear model of an atom in which he explained that a nucleus positively charged is surrounded by negatively charged electrons.
The model has a special place in the history of physics because it introduced an early quantum theory which brought about new developments in scientific thought and later culminated in the development of quantum mechanics. Thus the electrons would lose energy and spiral into. In other words Bohrs work sug.
Two dimensional toy model cannot represent the real world. Try out different models by shooting light at the atom. This is often expressed in terms of the inverse wavelength or wave number as follows.
The reason for the variation of R is that for hydrogen the mass of the orbiting electron is not negligible compared to. Electrons are distributed uniformly throughout the atom. Bohrs model required only one assumption.
Bohrs theory is not quantum mechanical but Schrödingers theory is. Bohr model of the atom was proposed by Neil Bohr in 1915. In 1913 Niels Bohr obtained the energy levels and spectral frequencies of the hydrogen atom after making a number of simple assumptions in order to correct the failed classical model.
Bohr thought that electrons orbited the nucleus in circular paths. Bohr was able to predict the difference in energy between each energy level allowing us to predict the energies of each line in the emission spectrum of hydrogen and understand why electron energies are quantized.
Niels Bohr
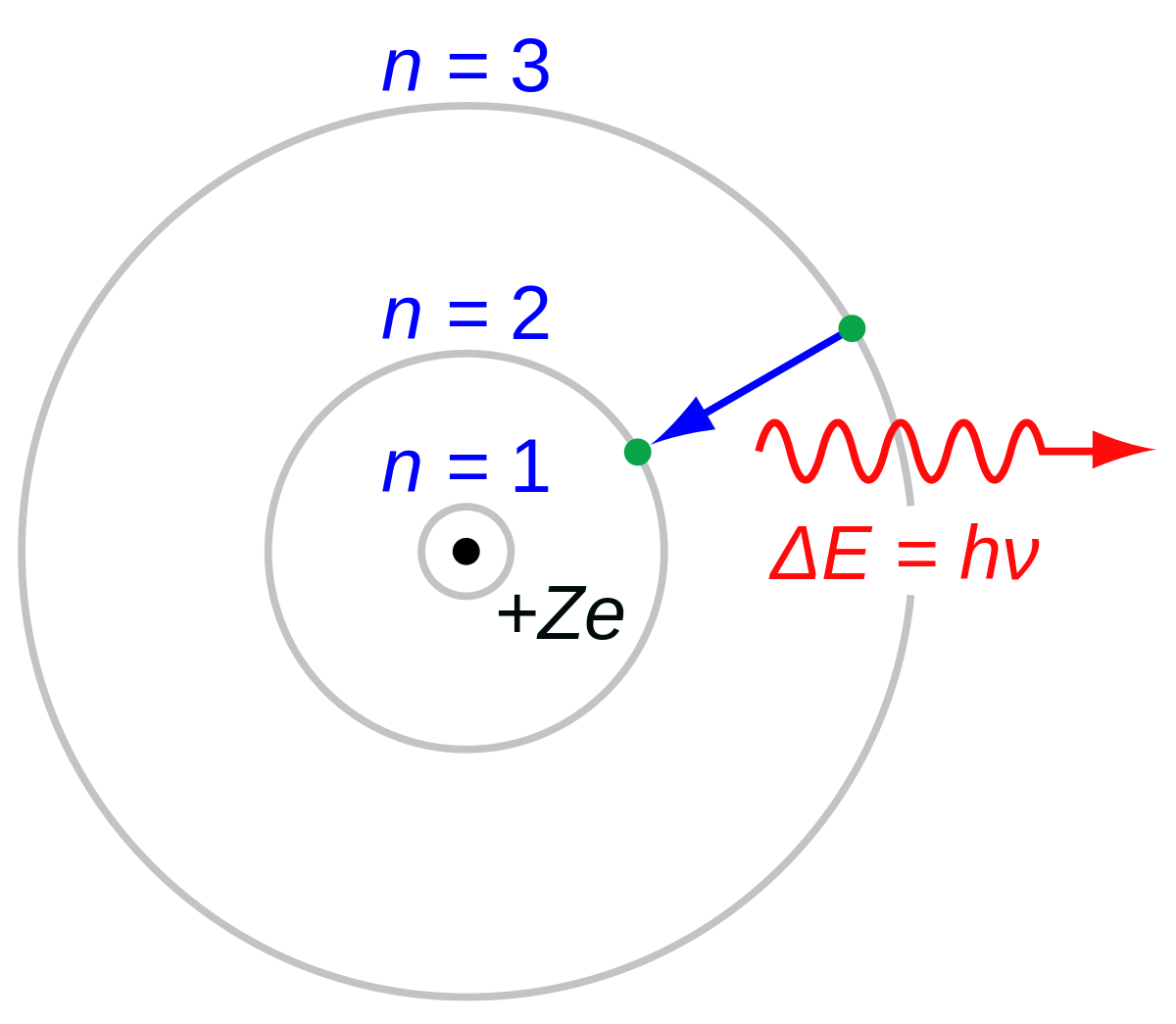
Bohr Model Wikipedia

Bohr Model Of Scientific Hydrogen Atom Royalty Free Vector
Bohr Model Of The Atom Overview And Examples

Pdf Bohr Model Of The Hydrogen Atom

Bohr Model Wikipedia
Bohr Model Of Hydrogen Atom Definition And Properties
Bohr Model Wikipedia




0 Response to "Bohr Model Of Hydrogen Atom"
Post a Comment