Group 18 Periodic Table
14 Electronic Structure of Atoms. The f-block columns between groups 2 and 3 are not numbered.

Noble Gas Definition Elements Properties Characteristics Facts Britannica
Periodic Table Noble Gases Group 18 Stock Photo Download Image Now Istock
There is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties.

Group 18 periodic table. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms ie the same core charge because. Social and Applied Aspects. Interactive periodic table of elements - your complete guide to the elements including definition mass names of each chemical in the periodic table.
Group 3A or IIIA of the periodic table includes the metalloid boron B as well as the metals aluminum Al gallium Ga indium In and thallium TlBoron forms mostly covalent bonds while the other elements in Group 3A form mostly ionic bonds. The elements can also be classified into the main-group elements or representative elements in the columns labeled 1 2 and 1318. This periodic table guide breaks down the elements name.
In 1869 Russian chemist Dmitri Mendeleev noticed there existed an innate pattern of. Group A vertical column in the periodic table. In other words there are 18 groups in modern periodic table Now Ill tell you amazing things about Periods of the Periodic table.
There are total 18 vertical columns in modern periodic table. P-block is group 3A to 8A These are the representative elements or main group elements. Alkaline earth metals or beryllium family.
The transition metals in the columns labeled 312. Inorganic Ventures provides an interactive periodic table. The periodic table also known as the periodic table of the chemical elements is a tabular display of the chemical elementsIt is widely used in chemistry physics and other sciences and is generally seen as an icon of chemistryIt is a graphic formulation of the periodic law which states that the properties of the chemical elements exhibit a periodic dependence on their atomic numbers.
And inner transition metals in the two rows at the bottom of the table the top-row elements are called lanthanides and the bottom-row elements are actinides. The vertical columns in the periodic table are known as groups of periodic table. Name Atomic Number Atomic Mass Electron Configuration Number of Neutrons Melting Point Boiling Point Date of Discovery Crystal Structure.
Periodic table Groups. Get essential facts about the first 20 elements all in one convenient place including the name atomic number atomic mass element symbol group and electron configurationIf you need detailed facts about these elements or any of the higher numbered ones start with the. So you have seen the above periodic table labeled with group names from 1-18.
Number of valence electrons c. All of the 1A elements have one valence electron. The Group 3A metals have three valence electrons in their highest-energy orbitals ns 2 p 1.
D-block is group 3B to 2B look at the periodic table above because the numbers are not sequential These are the transition metals. Alkali metals or lithium family. Elements in the same group have the same number of valence electrons.
There are total 18 groups vertical columns on the periodic table. Use the buttons above to change your view of the periodic table and view Murray Robertsons stunning Visual Elements artwork. Home About This Site Comments Help Links Window Version.
The periodic table is a masterpiece of organised chemical information and the evolution of chemistrys periodic table into the current form is an astonishing achievement. In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elementsThere are 18 numbered groups in the periodic table. See About the Periodic Table for information on how Group can be used to characterize an element.
Elements of the same group column have the same valence electrons or configuration. Z atomic number number of protons in the nucleus number of electrons orbiting the nucleus. It is organized in order of increasing atomic number.
A group is any column on the periodic tableElements in the same group usually have similar properties because they have the same number of electrons in the outermost electron shellThere are eight main groups of elements numbered 1 2 and 13-18. Group 1 Period 2. The Periodic Table of Elements ELEMENTS IN SAME COLUMN GROUP HAVE SIMILAR CHEMICAL PROPERTIES.
Periodic table and atomic structure. A group is a vertical column of the periodic table. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass.
This is what is. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell.
Alkali Metals Alkaline Earth. The standard form of the periodic table shown here includes periods shown horizontally and groups shown vertically. The periodic table as a list of elements arranged so as to demonstrate trends in their physical and chemical properties.
Meanwhile elements in the same period have the same number of occupied electron shells. The atomic number of each element increases by one reading from left to right. The periodic table is organized into groups vertical columns periods horizontal rows and families groups of elements that are similar.
Period A horizontal row in the periodic table. Back to Elements List. The periodic table is a tabular arrangement of the chemical elements.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. Click the tabs at the top to explore each section. S-block is group 1A 2A.
Families of the Periodic Table. The periodic table arranges the elements with their diverse physical and chemical properties in order of atomic number and fits them into a logical pattern. We have seen that the horizontal rows of periodic table are known as periods.
An up-to-date periodic table with detailed but easy to understand information. Eighteen columns divide the elements into groups with closely related physical properties. One reason the periodic table of the elements is so useful is that it is a means of arranging elements according to their similar properties.
First ionization energy general trends b. The Royal Society of Chemistrys interactive periodic table features history alchemy podcasts videos and data trends across the periodic table. Remember that Mendeleev arranged the periodic table so that elements with the most similar properties were placed in the same group.
For main group elements select ALL properties that decrease from top to bottom in a group on the periodic table. Comparison of Mendeleevs table with the modern periodic table. The name of each element in brown is accompanied by its chemical symbol in red as well as its atomic number Z and its most common or most stable mass number A.

Group 0 18 Noble Gases Physical Properties Uses Helium Neon Argon Krpton Xenon Radon Melting Points Boiling Points Atomic Radii Density Inertness Explained Gcse Chemistry Ks4 Science Igcse O Level Revision Notes

Chemistry Periodic Table By James Rowland
W3spoint Com
Groups Of The Periodic Table Video Khan Academy
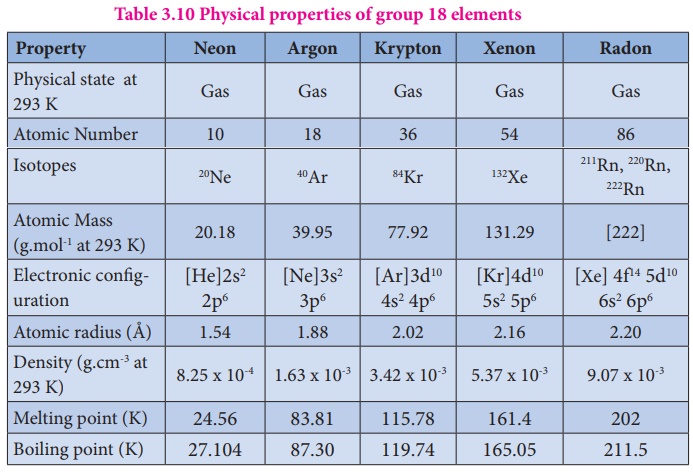
Group 18 Inert Gases Elements Occurrence Preparation Properties Structure Uses

Group 18 Elements Periodic Table Youtube
Chemistry S Ever Useful Periodic Table Celebrates A Big Birthday Science News For Students
Reactions Of Main Group Elements With Hydrogen Chemistry Libretexts



0 Response to "Group 18 Periodic Table"
Post a Comment