Citric Acid Pka Values
Hydrochloric Acid - HCl 0-2. But in general lemon juice is highly acidic with a pH of 2 because of the high percentage of acid it contains.
Acid Dissociation Constant Wikipedia

Ionization Equations And Acid Dissociation Constants Of Citric Acid And Download Table

Citric Acid An Overview Sciencedirect Topics
Required pH 5 pka 47 Molar concentration of acid required 1M Molar concentration of base required x M.

Citric acid pka values. Density of aqueous solutions of organic acids Changes in density of aqueous solutions with changes in concentration at 20C. Citric acid is a weak acid with a pKa of 279. When a compound has multiple pKa values a larger pH range becomes available for a buffer.
According to Paulings first rule successive pK values of a given acid increase pK a2 pK a1. Benzoic acid is a monoprotic acid with pKa 420. At higher pH values more than half will be ionized.
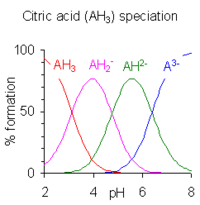
Lemon juice contains high levels of citric acid with around 05 grams per milliliter. PKa_1 215 pKa_2 720 pKa_3 12. It has a role as a food acidity regulator a chelator an antimicrobial agent and a fundamental metabolite.
Williams page-1 pKa Values INDEX Inorganic 2 Phenazine 24 Phosphates 3 Pyridine 25 Carboxylic acids 4 8 Pyrazine 26 Aliphatic 4 8 Aromatic 7 8 Quinoline 27. Because of the very large range of acid strengths greater than 10 40 a logarithmic scale of acidity pK a is normally employedStronger acids have smaller or more negative pK a values than do weaker acids. The pK_b values for the dibasic base B.
A pKa Database equilibrium constants of some 250 acids and bases see list have been compiled into a database expandable by the user. Buffers pKa range. Is a triprotic acid with the following pKa values.
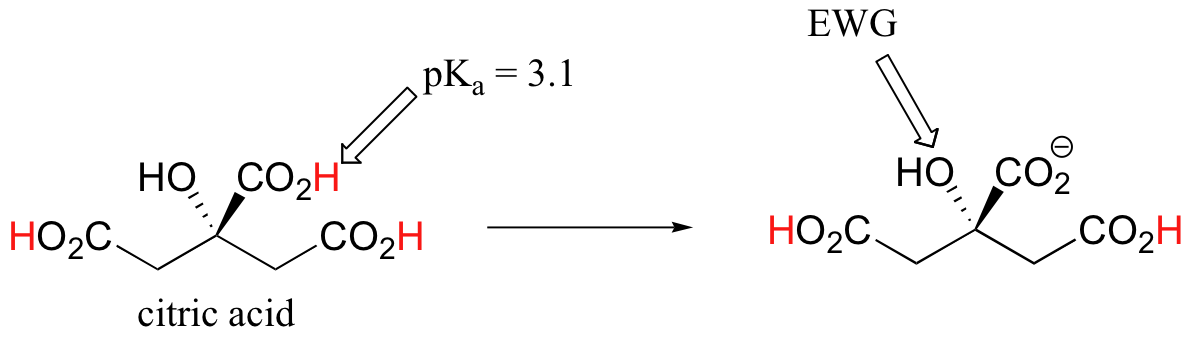
Nitric Acid - HNO. Citric acid has three carboxylic acid groups three ionizable acidic hydrogen atoms and three KapKa values. The neutralization reaction with sodium hydroxide has 3 to 1 stoichiometry as illustrated by the balanced complete neutralization equation.
Density of acetic acid citric acid formic acid D-lactic acid oxalic acid and trichloroacetic acid in water is plotted as function of wt molkg water and moll solution. PH PH measures the concentration of H ions or H3O ions of the citric acid solution. The value of K a is used to calculate the pH of weak acidsThe pK a value is used to choose a buffer when needed.
So by putting above information in equation we get. Citric acid also dissolves in absolute anhydrous ethanol 76 parts of citric acid per 100 parts of ethanol at 15 C. PH pka logSalt Acid 5 47 log x 1 5 47 log x log 1 as log 1 0 03 log x x log- 03 log- means anti-log x 2 Result in order to prepare buffer solution of pH 5 acetic acid CH3COOH.
Choosing an acid or base where pK a is close to the pH needed gives the best results. The pK a values given here are extrapolated for water at 25 ºC. The smaller the difference the more the overlap.
Citric acid is a weak organic acid containing three carboxylic acid functional groups and as a result it has three PKa values PKa1 314 PKa2 477 and PKa3 639. Density of acetic acid citric acid formic acid D-lactic acid oxalic acid and trichloroacetic acid in water is plotted as function of wt molkg water and moll solution. HOClO 3 pK a -8 HOClO 2 pKa -10 HOClO pKa 192 HOCl pKa 753.
The strongest acid is perchloric acid on the left and the weakest is hypochlorous acid on the far right. Azelaic acid is a saturated dicarboxylic acid found naturally in wheat rye and barley. Citric acid is a tricarboxylic acid that is propane-123-tricarboxylic acid bearing a hydroxy substituent at position 2.
Potassium Chloride KCl 11-18. Lets use an ICE table to calculate the. This means that at pH lower than acetic acids pKa less than half will be dissociated or ionized.
Density of aqueous solutions of some inorganic substances - Changes in density of aqueous solutions with changes in concentration at 20C. Oxalic Acid C. The exact level of citric acid in lemon juice can fluctuate.
Ideally we should do a systematic calculation involving all three acidity constants. Buffer pKa and pH Range Values For preparation of. It decomposes with loss of carbon dioxide above about 175 C.
Williams pKa Values INDEX Inorganic 2 Phenazine 24 Phosphates 3 Pyridine 25 Carboxylic acids 4 8 Pyrazine 26 Aliphatic 4 8 Aromatic 7 8 Quinoline 27 Phenols 9 Quinazoline 27 Alcohols and oxygen acids 10 11 Quinoxaline 27 Amino Acids 12 Special Nitrogen Compounds 28 Peptides 13 Hydroxylamines 28 Nitrogen Compounds 14. PKa is used to describe the acid dissociation. Table of Acids with Ka and pKa Values CLAS Acid HA A-Ka pKa Acid Strength Conjugate Base Strength Hydroiodic HI I-Hydrobromic HBr Br-Perchloric HClO4 ClO4-Hydrochloric HCl Cl-Chloric HClO3 ClO3-Sulfuric 1 H2SO4 HSO4-Nitric HNO3 NO3-Strong acids completely dissociate in aq solution Ka.
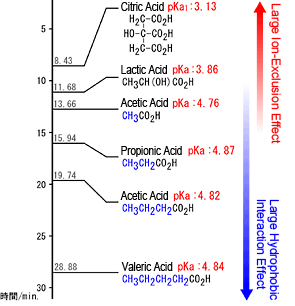
Generally any acid with a pKa -logK a less than about -2 is said to be a strong acid. Citric acid is a tribasic acid with pK a values extrapolated to zero ionic strength of 3128 4761 and 6396 at 25 C. Perchloric Acid HClO.
There are others that are not so common but I wouldnt expect you to know those unless I gave you their K a value. Citric 309 475 541 6 Br 3- 066 20 Crotonic 469 6 Cl 3- 065 20 Dihydroxyfumaric 114 6 F. Quick load of pKa sets of seven acid-base systems into the pH_calc Simulation and Regression modules for simultaneous use.
However we can get a pretty good approximate answer by assuming that only the first ionization is important. Many of the pK a values given for weak carbon acids are. Citric acid is a weak triprotic acid.
Its also possible to combine buffers providing their pKa values are close differing by 2 or less and adjusting the pH with strong base or acid to reach the required range. The case of citric acid is shown at the right. K a is the equilibrium constant for the dissociation reaction of a weak acidA weak acid is one that only partially dissociates in water or an aqueous solution.
An exception is citric acid because it has three pKa values. Buffers in the pH. Values of K a for.
Indeed if you set A HA you find that the pKa of an acid is simply the pH at which half of the acid is dissociated and half is intact. Solutions of citric acid are buffered over the whole range of pH 25 to 75. PKa Data Compiled by R.
It is also produced by Malassezia furfur also known as Pityrosporum ovale which is a species of fungus that is normally found on human skinAzelaic acid is effective against a number of skin conditions such as mild to moderate acne when applied topically in a cream formulation of 20. However you can calculate the pH of an aqueous solution of citric acid. What mass of citric acid and monosodium citrate must be added to 2 l to produce a1 M citrate buffer at.
PKa Data Compiled by R. Determine the equivalence points for a titration of 500mL of 0150M Citric acid with 0200M. It is an important metabolite in the pathway of all aerobic organisms.
HCl - hydrochloric acid H 2 SO 4 - sulfuric acid HClO 4 - perchloric acid HBr - hydrobromic acid HI - hydroiodic acid. Consider the family of chlorooxoacids which are arranged below in order of pK a values. A discussion of acid-base terminology is available here.
Definitions and values of dissociation constants for weak and strong acids and bases - KOH NaOH HCl H2SO4 HClO4 HNO3 CaOH2 and other About us Feedback pH calculator program - Base Acid Titration and Equilibria - dissociation constants pKa and pKb.
7 6 Acidos Poliproticos Libretexts Espanol

Table 1 From Citric Acid Adsorption On Tio2 Nanoparticles In Aqueous Suspensions At Acidic And Circumneutral Ph Surface Coverage Surface Speciation And Its Impact On Nanoparticle Nanoparticle Interactions Semantic Scholar
Analytical Methods For Organic Acids Shimadzu Shimadzu Corporation
Citric Acid Has 3 Pka Values 3 128 4 761 Chegg Com

Effects Of Speciation On The Physical Properties Of Frozen Solutions American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology

A Chemical Structure And P Ka Values Of Citric Acid B Download Scientific Diagram
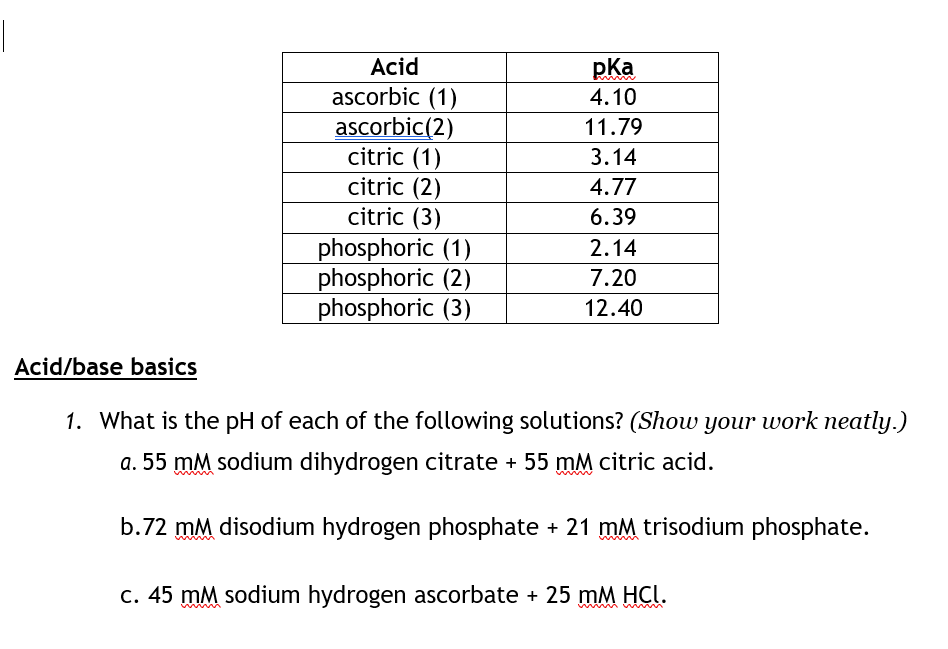
Solved Acid Ascorbic 1 Ascorbic 2 Citric 1 Citric 2 Chegg Com

Solved Question 7 2 Points Icitric Acid H3cshsoz Has Pka Values Of 3 128 Pka1 4 761 Pka2 Ad 16 396 Pka3 Iwhat Is The Predominant Form Of Citric Acid At Ph 4 373 Hscohsoz Ohzcshsoz Hcohsoz Dchsoz
0 Response to "Citric Acid Pka Values"
Post a Comment