Atm To Mmhg
Multiply the atm value by 7600 mmHg atm. Atm x 760 mmHg Calculations.

Solved Gas Pressures Can Be Expressed In Units Of Mmhg Atm Chegg Com

Converting Between Units Of Pressure And
Die Einheiten geben den statischen Druck an der von.
Atm to mmhg. 1 atm 101325 kPa atm to kPa. Comprehensive user-friendly humidity calculator supporting all popular parameters including dewfrost-point rh ppmV moisture content and more. If 600 L of nitrogen is collected over water at 400 C when the atmospheric pressure is 7600 mm Hg what is the partial pressure of the nitrogen.
1 atmosphere is equal to how many mmHg. Under normal conditions when the pressure of the atmosphere is approximately 760 mmHg water boils at 100 o C. The valve between the 200 L bulb in which the gas pressure is 100 atm and the 300 L bulb in which the gas pressure is 150 atm is opened.
Calculate the partial pressure of helium and argon if the total pressure inside the container is 400 atm. Convert 8 atmospheres to mmHg show work Formula. 1 atm 146959 psi atm to psi.
975 mmHg is equal to 128289474 atm Conversion Table For quick reference purposes below is a conversion table that you can use to convert from mmHg to atm. Atm or mmHg The SI derived unit for pressure is the pascal. Diver must alter their habits according to the pressure they experience.
1 bar 0986923 atm. Bar is a metric pressure unit and equals to 100 kilopascals which is almost equal to the atmospheric pressure. You can view more details on each measurement unit.
1 atm 760 mmHg 760 Torr. 1 atm 101325 pascals atm to pascal. The answer is 7599998769899.
It is denoted mmHg or mm Hg. We assume you are converting between atmosphere standard and millimeter of mercury 0 C. MmHg 760 atm Calculations.
1 atm to mmHg. If you use the first value of R which is 0082057 L atm mol-1 K-1 your unit for pressure must be atm for volume must be liter for temperature must be Kelvin. Torr or millimeter of mercury mmHg is a non-SI unit of pressure.
It is a comparative measure of how hot or cold a material is. The standard atmosphere was originally defined as the pressure exerted by 760 mm of mercury at 0 C and standard gravity g n 9806 65 ms 2. How many atm in 1 mmHg.
1 atm 760 torr mmHg atm to torr. A millimetre of mercury is a manometric unit of pressure formerly defined as the extra pressure generated by a column of mercury one millimetre high and currently defined as exactly 133322 387 415 pascals. 975 mmHg 760 128289474 atm Result.
Mm Hg sind identische Maßeinheiten des Druckes. Latmosphère normale symbole atm est une unité de pression qui nappartient pas au Système international SI. At atmosphère technique atm atmosphère normale bar Bar cm Centimètres ft Pieds feets in Pouces inches inH2O Pouce colonne deau inHg Pouce colonne de mercure kp Kiloponds kPa Kilopascal.
The answer is 00013157896866519. Although not an SI unit the millimetre of mercury is still routinely used in medicine meteorology aviation and many other scientific fields. Elle a été définie lors de la 10 e Conférence générale des poids et mesures en 1954 comme étant égale à 1 013 250 dyncm 2 soit 101 325 Pa 1Elle correspond à la pression dune hauteur de 760 mmHg à 0 C sous laccélération normale g 9806 65 ms 2.
How much 1 mmHg is equal to atm. The standard constant value used for atmospheric pressure at sea level is 1 atm standard atmosphere which equals 101325 pascals in SI units and is equivalent to 299213 inches of mercury. The normal boiling point of water is 100 o C because this is the temperature at which the vapor pressure of water is 760 mmHg or 1 atm.
62363 6711 L mmHg K1 mol1 62363 6711 L Torr K1 mol1 6132 44010 ft lbf K1 g-mol1 1545348 963 ft lbf R1 lb-mol1 10731 592 ft3 psi R1 lb-mol1 0730 241312 ft3 atm R1 lb-mol1 1314 43 ft3 atm K1 lb-mol1 998970117 ft3 mmHg K1 lb-mol1. 1 l atm 101325 joules J 1 l atm 0101325 kilojoules kJ 1 l atm 24217256214149 calories cal 1 l atm 0024217256214149 kilocalories kcal 1 l atm 63241957832453E20 electron volt eV 1 l atm 0028145833333333 watt hour Wh 1 l atm 23241043510812E19 atomic unit of energy au 1 l atm 24217256214149E-8 tons of TNT tTNT. 1 pascal is equal to 98692326671601E-6 atm or 00075006156130264 mmHg.
1 atm 101325 bar. Solution using Boyles Law. Lbf livre-force pounds force mbar Millibar mca Metros columna agua espagnol mce Mètres colonne deau mH2O Mètres colonne deau mm Milimètres mmca Milímetros columna agua espagnol.
At 10000 feet above sea level the pressure of the atmosphere is only 526 mmHg. Atmospheres to mmHg Conversion Chart. The coldest theoretical temperature is called absolute zeroIt is the temperature where the thermal motion of particles is at its minimum not the same as motionless.
It is denoted by mmHg or mm Hg. Temperature is the property of matter which reflects the quantity of energy of motion of the component particles. P Ar 400 - 120.
Atmospheric pressure also known as barometric pressure is the pressure within the atmosphere of EarthThe standard atmosphere is a unit of pressure. 8 atm x 760 6080 mmHg Result. You can view more details on each measurement unit.
It was used as a reference condition for physical and chemical properties and was implicit in the definition of the centigrade scale of temperature which defined 100 C as the boiling point of water at this pressure. 1 atm 0101325 MPa atm to MPa. 1 atm 10332274527999 m c.
Equals 7600 mm Hg so there will be a multiplication or division based on the direction of the change. 1 pascal is equal to 00075006156130264 mmHg or 98692326671601E-6 atm. 8 atm is equal to 6080 mmHg.
P He 0300 x 400 atm 120 atm. Convert 0875 atm to mmHg. The unit is named after Evangelista Torricelli Italian physicist and mathematician for his discovery of the principle of the barometer in 1643.
MmHg or atm The SI derived unit for pressure is the pascal. 1 Liter Atmosphere to common energy units. Metros de columna de agua 1 atm 1469594877551 psi.
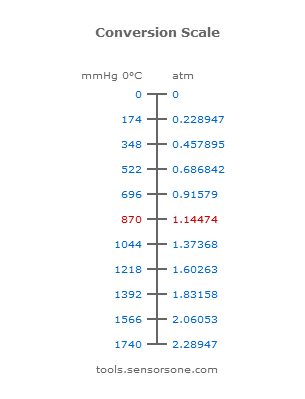
1 P 1 V 1 P 2 V 2 twice 100 atm 200 L x 500 L x 0. The standard atmosphere is denoted by atm. For quick reference purposes below is a conversion table that you can use to convert from atm to mmHg.
How many mmHg in 1 atm. Convert 975 mmHg to atmospheres show work Formula. Torr und die Millimeter-Quecksilbersäule Einheitenzeichen.
Assuming the density of sea water to be 1025 kgm³ in fact it is slightly variable pressure increases by 1 atm with each 10 m of depth. It is the atmospheric pressure that supports a column of mercury 1 millimeter high. 7600 mmHg minus 553.
What is the final pressure in the two bulbs the temperature being constant and the same in both bulbs. Die Einheit Millimeter-Quecksilbersäule teilweise geschrieben Millimeter Quecksilbersäule wird auch kurz Torr genannt zu Ehren Torricellis der das Quecksilberbarometer erfand. 1 atm 101325 Pa.
If you use the second value of R which is 62364 L Torr mol -1 K -1 your unit for pressure must be Torr for volume must be liter and for temperature must be Kelvin. 1 atmosphere atm 760000 mmHg millimetres of Mercury Average atmospheric pressure at sea level. We assume you are converting between millimeter of mercury 0 C and atmosphere standard.

Pressure Measurement

Solved Pes Patm P 0 974 Atm X 760 Mmhg 1 Atm 32 6 Chegg Com
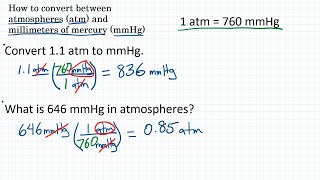
How To Convert Pressure Units Atm Mmhg Youtube

Mmhg Millimetres Of Mercury At 0 Deg C Pressure Unit

1 000 105 Kpa Atm 5 914 10 6 Atm Mmhg Kpa Brainly Com

Chapter 6 Gases 6 1 Properties Of Gases Ppt Download

Gases Unit 10 Kinetic Molecular Theory Of Gases 1 Gases Consist Of Tiny Atoms Or Molecules That Are In Constant Random Motion 2 The Space Between Gas Ppt Download

Pressure Units And Conversions Chemistry For Non Majors

0 Response to "Atm To Mmhg"
Post a Comment